Introductory Chemistry: A Foundation - Zumdahl S.S., DeCoste D.J. 2019
Reactions in Aqueous Solutions
Reactions in Which a Solid Forms
Objective
· To learn to identify the solid that forms in a precipitation reaction.
One driving force for a chemical reaction is the formation of a solid, a process called precipitation . The solid that forms is called a precipitate , and the reaction is known as a precipitation reaction . For example, when an aqueous (water) solution of potassium chromate, , which is yellow, is added to a colorless aqueous solution containing barium nitrate, , a yellow solid forms (Fig. 7.1). The fact that a solid forms tells us that a reaction—a chemical change—has occurred. That is, we have a situation where
Figure 7.1.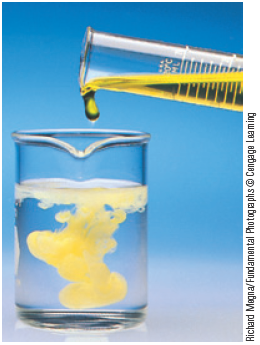
Richard Megna/Fundamental Photographs © Cengage Learning
The precipitation reaction that occurs when yellow potassium chromate, , is mixed with a colorless barium nitrate solution, .
What is the equation that describes this chemical change? To write the equation, we must decipher the identities of the reactants and products. The reactants have already been described: and . Is there some way in which we can predict the identities of the products? What is the yellow solid? The best way to predict the identity of this solid is to first consider what products are possible. To do this we need to know what chemical species are present in the solution that results when the reactant solutions are mixed. First, let’s think about the nature of each reactant in an aqueous solution.
What Happens When an Ionic Compound Dissolves in Water?
The designation means that barium nitrate (a white solid) has been dissolved in water. Note from its formula that barium nitrate contains the and ions. In virtually every case when a solid containing ions dissolves in water, the ions separate and move around independently. That is, does not contain units. Rather, it contains separated and ions. In the solution there are two ions for every ion. Chemists know that separated ions are present in this solution because it is an excellent conductor of electricity (Fig. 7.2). Pure water does not conduct an electric current. Ions must be present in water for a current to flow.
Figure 7.2.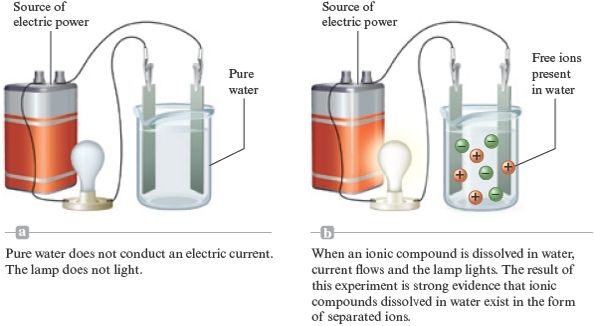
Electrical conductivity of aqueous solutions.
When each unit of a substance that dissolves in water produces separated ions, the substance is called a strong electrolyte . Barium nitrate is a strong electrolyte in water because each unit produces the separated ions ( , , ).
Similarly, aqueous also behaves as a strong electrolyte. Potassium chromate contains the and ions, so an aqueous solution of potassium chromate (which is prepared by dissolving solid in water) contains these separated ions. That is, does not contain units but instead contains cations and anions, which move around independently. (There are two ions for each ion.)
The idea introduced here is very important: when ionic compounds dissolve, the resulting solution contains the separated ions. Therefore, we can represent the mixing of and in two ways. We usually write these reactants as
However, a more accurate representation of the situation is
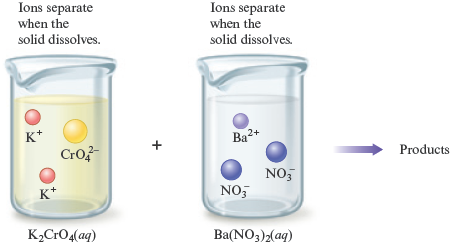
We can express this information in equation form as follows:
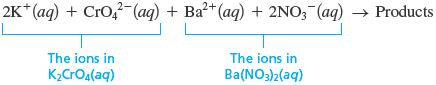
Thus the mixed solution contains four types of ions: , , , and . Now that we know what the reactants are, we can make some educated guesses about the possible products.
How to Decide What Products Form
Which of these ions combine to form the yellow solid observed when the original solutions are mixed? This is not an easy question to answer. Even an experienced chemist is not sure what will happen in a new reaction. The chemist tries to think of the various possibilities, considers the likelihood of each possibility, and then makes a prediction (an educated guess). Only after identifying each product experimentally can the chemist be sure what reaction actually has taken place. However, an educated guess is very useful because it indicates what kinds of products are most likely. It gives us a place to start. So the best way to proceed is first to think of the various possibilities and then to decide which of them is most likely.
What are the possible products of the reaction between and or, more accurately, what reaction can occur among the ions , , , and ? We already know some things that will help us decide. We know that a solid compound must have a zero net charge. This means that the product of our reaction must contain both anions and cations (negative and positive ions). For example, and could not combine to form the solid because such a solid would have a positive charge. Similarly, and could not combine to form a solid because that solid would have a negative charge.
Something else that will help us is an observation that chemists have made by examining many compounds: most ionic materials contain only two types of ions—one type of cation and one type of anion. This idea is illustrated by the following compounds (among many others):
Compound |
Cation |
Anion |
All the possible combinations of a cation and an anion to form uncharged compounds from among the ions , , , and are shown in the following table:
So the compounds that might make up the solid are
Which of these possibilities is most likely to represent the yellow solid? We know it’s not or ; these are the reactants. They were present (dissolved) in the separate solutions that were mixed initially. The only real possibilities are and . To decide which of these is more likely to represent the yellow solid, we need more facts. An experienced chemist, for example, knows that is a white solid. On the other hand, the ion is yellow. Therefore, the yellow solid most likely is .
We have determined that one product of the reaction between and is , but what happened to the and ions? The answer is that these ions are left dissolved in the solution. That is, does not form a solid when the and ions are present in water. In other words, if we took the white solid and put it in water, it would totally dissolve (the white solid would “disappear,” yielding a colorless solution). So when we mix and , forms but is left behind in solution [we write it as ]. (If we poured the mixture through a filter to remove the solid and then evaporated all of the water, we would obtain the white solid .)
After all this thinking, we can finally write the unbalanced equation for the precipitation reaction:
We can represent this reaction in pictures as follows:
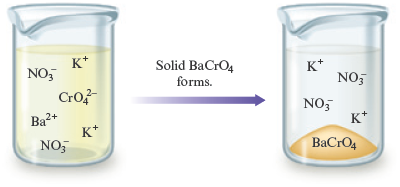
Note that the and ions are not involved in the chemical change. They remain dispersed in the water before and after the reaction.
Using Solubility Rules
In the previous example we were finally able to identify the products of the reaction by using two types of chemical knowledge:
1. Knowledge of facts
2. Knowledge of concepts
For example, knowing the colors of the various compounds proved very helpful. This represents factual knowledge. Awareness of the concept that solids always have a net charge of zero was also essential. These two kinds of knowledge allowed us to make a good guess about the identity of the solid that formed. As you continue to study chemistry, you will see that a balance of factual and conceptual knowledge is always required. You must both memorize important facts and understand crucial concepts to succeed.
In the present case we are dealing with a reaction in which an ionic solid forms—that is, a process in which ions that are dissolved in water combine to give a solid. We know that for a solid to form, both positive and negative ions must be present in relative numbers that give zero net charge. However, oppositely charged ions in water do not always react to form a solid, as we have seen for and . In addition, and can coexist in water in very large numbers with no formation of solid . In other words, when solid (common salt) is placed in water, it dissolves—the white solid “disappears” as the and ions are dispersed throughout the water. (You probably have observed this phenomenon in preparing salt water to cook food.) The following two statements, then, are really saying the same thing.
1. Solid is very soluble in water.
2. Solid does not form when one solution containing is mixed with another solution containing .
To predict whether a given pair of dissolved ions will form a solid when mixed, we must know some facts about the solubilities of various types of ionic compounds. In this text we will use the term soluble solid to mean a solid that readily dissolves in water; the solid “disappears” as the ions are dispersed in the water. The terms insoluble solid and slightly soluble solid are taken to mean the same thing: a solid where such a tiny amount dissolves in water that it is undetectable with the naked eye. The solubility information about common solids that is summarized in Table 7.1 is based on observations of the behavior of many compounds. This is factual knowledge that you will need to predict what will happen in chemical reactions where a solid might form. This information is summarized in Fig. 7.3.
Table 7.1. General Rules for Solubility of Ionic Compounds (Salts) in Water at
1. |
Most nitrate salts are soluble. |
2. |
Most salts of , , and are soluble. |
3. |
Most chloride salts are soluble. Notable exceptions are , , and . |
4. |
Most sulfate salts are soluble. Notable exceptions are , , and . |
5. |
Most hydroxide compounds are only slightly soluble.* The important exceptions are and . and are only moderately soluble. |
6. |
Most sulfide , carbonate , and phosphate salts are only slightly soluble.* |
Figure 7.3.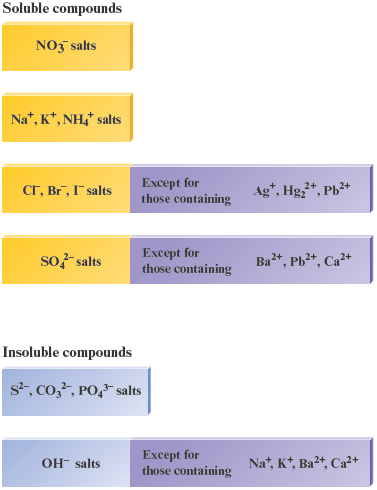
Solubilities of common compounds.
Notice that in Table 7.1 and Fig. 7.3 the term salt is used to mean ionic compound. Many chemists use the terms salt and ionic compound interchangeably. In Example 7.1, we will illustrate how to use the solubility rules to predict the products of reactions among ions.
Critical Thinking
· What if no ionic solids were soluble in water? Could reactions occur in aqueous solutions?
Interactive Example 7.1. Identifying Precipitates in Reactions Where a Solid Forms
When an aqueous solution of silver nitrate is added to an aqueous solution of potassium chloride, a white solid forms. Identify the white solid, and write the balanced equation for the reaction that occurs.
Solution
First let’s use the description of the reaction to represent what we know:
Remember, try to determine the essential facts from the words and represent these facts by symbols or diagrams. To answer the main question (What is the white solid?), we must establish what ions are present in the mixed solution. That is, we must know what the reactants are really like. Remember that when ionic substances dissolve in water, the ions separate. So we can write the equation
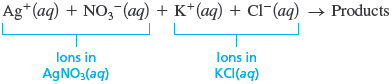
or using pictures
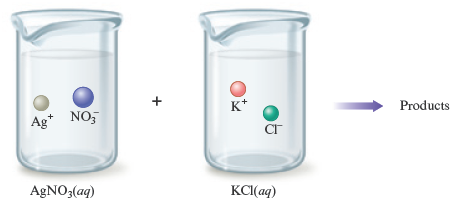
to represent the ions present in the mixed solution before any reaction occurs. In summary:
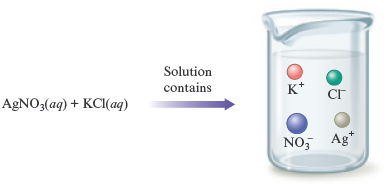
Now we will consider what solid might form from this collection of ions. Because the solid must contain both positive and negative ions, the possible compounds that can be assembled from this collection of ions are
and are the substances already dissolved in the reactant solutions, so we know that they do not represent the white solid product. We are left with two possibilities:
·
·
Another way to obtain these two possibilities is by ion interchange. This means that in the reaction of and , we take the cation from one reactant and combine it with the anion of the other reactant.
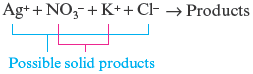
Ion interchange also leads to the following possible solids:
or
To decide whether or is the white solid, we need the solubility rules (see Table 7.1). Rule 2 states that most salts containing are soluble in water. Rule 1 says that most nitrate salts (those containing ) are soluble. So the salt is water-soluble. That is, when and are mixed in water, a solid does not form.
On the other hand, Rule 3 states that although most chloride salts (salts that contain ) are soluble, is an exception. That is, is insoluble in water. Thus the white solid must be . Now we can write
What is the other product?
To form , we have used the and ions:
![]()
This leaves the and ions. What do they do? Nothing. Because is very soluble in water (Rules 1 and 2), the and ions remain separate in the water; the remains dissolved, and we represent it as . We can now write the full equation:
Figure 7.4 shows the precipitation of that occurs when this reaction takes place. In graphic form, the reaction is
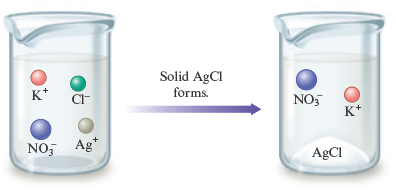 Figure 7.4.
Figure 7.4.
© Cengage Learning
Precipitation of silver chloride occurs when solutions of silver nitrate and potassium chloride are mixed. The and ions remain in solution.
The following strategy is useful for predicting what will occur when two solutions containing dissolved salts are mixed.
How to Predict Precipitates When Solutions of Two Ionic Compounds Are Mixed
Step 1.
Write the reactants as they actually exist before any reaction occurs. Remember that when a salt dissolves, its ions separate.
Step 2.
Consider the various solids that could form. To do this, simply exchange the anions of the added salts.
Step 3.
Use the solubility rules (Table 7.1) to decide whether a solid forms and, if so, to predict the identity of the solid.
Interactive Example 7.2. Using Solubility Rules to Predict the Products of Reactions
Using the solubility rules in Table 7.1, predict what will happen when the following solutions are mixed. Write the balanced equation for any reaction that occurs.
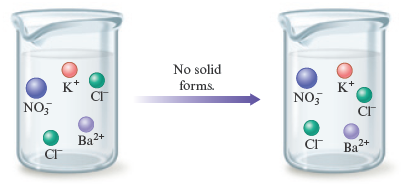
a. and
b. and
c. and
Solution (a)
Step 1.
represents an aqueous solution obtained by dissolving solid in water to give the ions and . Likewise, is a solution formed by dissolving solid in water to produce and . When these two solutions are mixed, the following ions will be present:

Step 2.
To get the possible products, we exchange the anions.
![]()
This yields the possibilities and . These are the solids that might form. Notice that two ions are needed to balance the charge on .
Step 3.
The rules listed in Table 7.1 indicate that both and are soluble in water. So no precipitate forms when and are mixed. All of the ions remain dissolved in the solution. This means that no reaction takes place. That is, no chemical change occurs.
Solution (b)
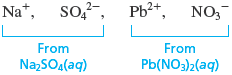
Step 1.
The following ions are present in the mixed solution before any reaction occurs:
Step 2.
Exchanging anions
![]()
yields the possible solid products and .
Step 3.
Using Table 7.1, we see that is soluble in water (Rules 1 and 2) but that is only slightly soluble (Rule 4). Thus, when these solutions are mixed, solid forms. The balanced reaction is
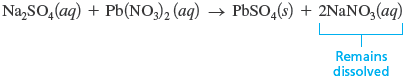
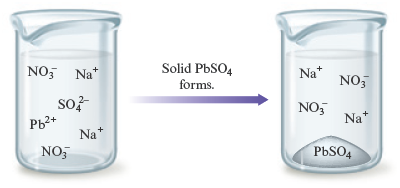
which can be represented as
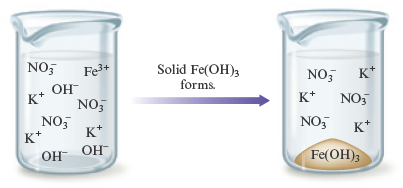
Solution (c)
Step 1.
The ions present in the mixed solution before any reaction occurs are
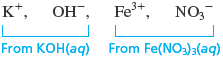
Step 2.
Exchanging anions
![]()
yields the possible solid products and .
Step 3.
Rules 1 and 2 (Table 7.1) state that is soluble, whereas is only slightly soluble (Rule 5). Thus, when these solutions are mixed, solid forms. The balanced equation for the reaction is
which can be represented as
Self-Check: Exercise 7.1
· Predict whether a solid will form when the following pairs of solutions are mixed. If so, identify the solid, and write the balanced equation for the reaction.
a. and
b. and
c. and
See Problems 7.17 and 7.18.