Introductory Chemistry: A Foundation - Zumdahl S.S., DeCoste D.J. 2019
Reactions in Aqueous Solutions
Reactions That Form Water: Acids and Bases
Objective
· To learn the key characteristics of the reactions between strong acids and strong bases.
In this section we encounter two very important classes of compounds: acids and bases. Acids were first associated with the sour taste of citrus fruits. In fact, the word acid comes from the Latin word acidus, which means “sour.” Vinegar tastes sour because it is a dilute solution of acetic acid; citric acid is responsible for the sour taste of a lemon. Bases, sometimes called alkalis, are characterized by their bitter taste and slippery feel, like wet soap. Most commercial preparations for unclogging drains are highly basic.
Acids have been known for hundreds of years. For example, the mineral acids sulfuric acid, , and nitric acid, , so named because they were originally obtained by the treatment of minerals, were discovered around 1300. However, it was not until the late 1800s that the essential nature of acids was discovered by Svante Arrhenius, then a Swedish graduate student in physics.
Arrhenius, who was trying to discover why only certain solutions could conduct an electric current, found that conductivity arose from the presence of ions. In his studies of solutions, Arrhenius observed that when the substances , , and were dissolved in water, they behaved as strong electrolytes. He suggested that this was the result of ionization reactions in water.
Arrhenius proposed that an acid is a substance that produces ions (protons) when it is dissolved in water.
Studies show that when , , and are placed in water, virtually every molecule dissociates to give ions. This means that when molecules of are dissolved in water, ions and ions are produced. Virtually no molecules exist in aqueous solution (Fig. 7.5). Because these substances are strong electrolytes that produce ions, they are called strong acids .
Figure 7.5.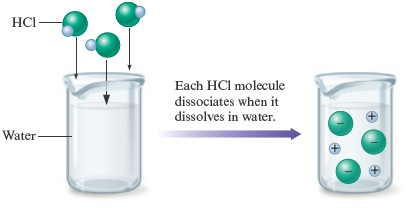
When gaseous is dissolved in water, each molecule dissociates to produce and ions. That is, behaves as a strong electrolyte.
Arrhenius also found that aqueous solutions that exhibit basic behavior always contain hydroxide ions. He defined a base as a substance that produces hydroxide ions in water. The base most commonly used in the chemical laboratory is sodium hydroxide, , which contains and ions and is very soluble in water.
Sodium hydroxide, like all ionic substances, produces separated cations and anions when it is dissolved in water.
Although dissolved sodium hydroxide is usually represented as , you should remember that the solution really contains separated and ions. In fact, for every units of dissolved in water, and ions are produced.
Potassium hydroxide has properties markedly similar to those of sodium hydroxide. It is very soluble in water and produces separated ions.
Because these hydroxide compounds are strong electrolytes that contain ions, they are called strong bases .
When strong acids and strong bases (hydroxides) are mixed, the fundamental chemical change that always occurs is that ions react with ions to form water.
Water is a very stable compound, as evidenced by the abundance of it on the earth’s surface. Therefore, when substances that can form water are mixed, there is a strong tendency for the reaction to occur. In particular, the hydroxide ion has a high affinity for ions, because water is produced in the reaction between these ions.
The tendency to form water is the second of the driving forces for reactions that we mentioned in Section 7.1. Any compound that produces ions in water reacts vigorously with any compound that can furnish ions to form . For example, the reaction between hydrochloric acid and aqueous sodium hydroxide is represented by the following molecular equation:
Because , , and exist as completely separated ions in water, the complete ionic equation for this reaction is
Notice that the and are spectator ions (they undergo no changes), so the net ionic equation is
Thus the only chemical change that occurs when these solutions are mixed is that water is formed from and ions.
Interactive Example 7.4. Writing Equations for Acid—Base Reactions
Nitric acid is a strong acid. Write the molecular, complete ionic, and net ionic equations for the reaction of aqueous nitric acid and aqueous potassium hydroxide.
Solution
Molecular equation:
Complete ionic equation:
Net ionic equation:
Note that and are spectator ions and that the formation of water is the driving force for this reaction.
There are two important things to note as we examine the reaction of hydrochloric acid with aqueous sodium hydroxide and the reaction of nitric acid with aqueous potassium hydroxide.
1. The net ionic equation is the same in both cases; water is formed.
2. Besides water, which is always a product of the reaction of an acid with , the second product is an ionic compound, which might precipitate or remain dissolved, depending on its solubility.
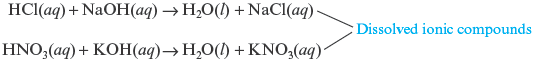
This ionic compound is called a salt . In the first case the salt is sodium chloride, and in the second case the salt is potassium nitrate. We can obtain these soluble salts in solid form (both are white solids) by evaporating the water.
Summary of Strong Acids and Strong Bases
The following points about strong acids and strong bases are particularly important.
1. The common strong acids are aqueous solutions of , , and .
2. A strong acid is a substance that completely dissociates (ionizes) in water. (Each molecule breaks up into an ion plus an anion.)
3. A strong base is a metal hydroxide compound that is very soluble in water. The most common strong bases are and , which completely break up into separated ions ( and or and ) when they are dissolved in water.
4. The net ionic equation for the reaction of a strong acid and a strong base (contains ) is always the same: it shows the production of water.
5. In the reaction of a strong acid and a strong base, one product is always water, and the other is always an ionic compound called a salt, which remains dissolved in the water. This salt can be obtained as a solid by evaporating the water.
6. The reaction of and is often called an acid—base reaction, where is the acidic ion and is the basic ion.