Introductory Chemistry: A Foundation - Zumdahl S.S., DeCoste D.J. 2019
Reactions in Aqueous Solutions
Reactions of Metals with Nonmetals (Oxidation—Reduction)
Objectives
· To learn the general characteristics of a reaction between a metal and a nonmetal.
· To understand electron transfer as a driving force for a chemical reaction.
In Chapter 4 we spent considerable time discussing ionic compounds—compounds formed in the reaction of a metal and a nonmetal. A typical example is sodium chloride, formed by the reaction of sodium metal and chlorine gas:
Let’s examine what happens in this reaction. Sodium metal is composed of sodium atoms, each of which has a net charge of zero. (The positive charges of the protons in its nucleus are exactly balanced by the negative charges on the electrons.) Similarly, the chlorine molecule consists of two uncharged chlorine atoms (each has protons and electrons). However, in the product (sodium chloride), the sodium is present as and the chlorine as . By what process do the neutral atoms become ions? The answer is that one electron is transferred from each sodium atom to each chlorine atom.
![]()
After the electron transfer, each sodium has electrons and protons (a net charge of ), and each chlorine has electrons and protons (a net charge of ).
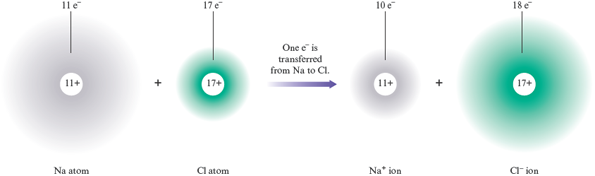
Thus the reaction of a metal with a nonmetal to form an ionic compound involves the transfer of one or more electrons from the metal (which forms a cation) to the nonmetal (which forms an anion). This tendency to transfer electrons from metals to nonmetals is the third driving force for reactions that we listed in Section 7.1. A reaction that involves a transfer of electrons is called an oxidation—reduction reaction .
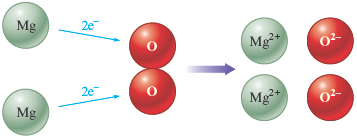
There are many examples of oxidation—reduction reactions in which a metal reacts with a nonmetal to form an ionic compound. Consider the reaction of magnesium metal with oxygen,
which produces a bright, white light that was once useful in camera flash units. Note that the reactants contain uncharged atoms, but the product contains ions:
Therefore, in this reaction, each magnesium atom loses two electrons and each oxygen atom gains two electrons . We might represent this reaction as follows:
Another example is
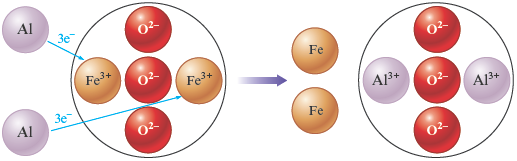
which is a reaction (called the thermite reaction) that produces so much energy (heat) that the iron is initially formed as a liquid (Fig. 7.6). In this case the aluminum is originally present as the elemental metal (which contains uncharged atoms) and ends up in , where it is present as cations (the ions just balance the charge of the ions). Therefore, in the reaction, each aluminum atom loses three electrons.
Figure 7.6.
© Cengage Learning
The thermite reaction gives off so much heat that the iron formed is molten.
The opposite process occurs with the iron, which is initially present as ions in and ends up as uncharged atoms in the elemental iron. Thus each iron cation gains three electrons to form an uncharged atom:
We can represent this reaction in schematic form as follows:
Example 7.5. Identifying Electron Transfer in Oxidation—Reduction Reactions
For each of the following reactions, show how electrons are gained and lost.
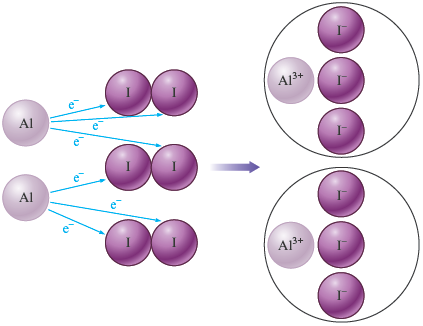
a. (This reaction is shown in Fig. 7.7. Note the purple “smoke,” which is excess being driven off by the heat.)
b. Figure 7.7.
© Cengage Learning
When powdered aluminum and iodine (shown in the foreground) are mixed (and a little water added), they react vigorously.
Solution
a. In the ions are and (aluminum always forms , and iodine always forms ). In the aluminum is present as uncharged atoms. Thus aluminum goes from to by losing three electrons . In each iodine atom is uncharged. Thus each iodine atom goes from to by gaining one electron . A schematic for this reaction is
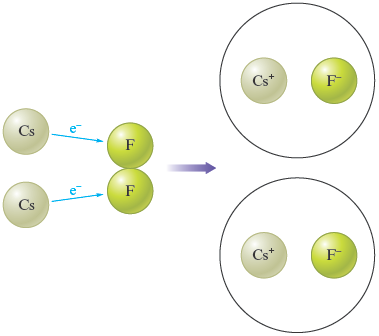
b. In the ions present are and . Cesium metal, , contains uncharged cesium atoms, and fluorine gas, , contains uncharged fluorine atoms. Thus in the reaction, each cesium atom loses one electron , and each fluorine atom gains one electron . The schematic for this reaction is
Self-Check: Exercise 7.3
· For each reaction, show how electrons are gained and lost.
a.
b.
See Problems 7.47 and 7.48.
So far we have emphasized electron transfer (oxidation—reduction) reactions that involve a metal and a nonmetal. Electron transfer reactions can also take place between two nonmetals. We will not discuss these reactions in detail here. All we will say at this point is that one sure sign of an oxidation—reduction reaction between nonmetals is the presence of oxygen, , as a reactant or product. In fact, oxidation got its name from oxygen. Thus the reactions
and
are electron transfer reactions, even though it is not obvious at this point.
We can summarize what we have learned about oxidation—reduction reactions as follows:
Characteristics of Oxidation—Reduction Reactions
1. When a metal reacts with a nonmetal, an ionic compound is formed. The ions are formed when the metal transfers one or more electrons to the nonmetal, the metal atom becoming a cation and the nonmetal atom becoming an anion. Therefore, a metal—nonmetal reaction can always be assumed to be an oxidation—reduction reaction, which involves electron transfer.
2. Two nonmetals can also undergo an oxidation—reduction reaction. At this point we can recognize these cases only by looking for as a reactant or product. When two nonmetals react, the compound formed is not ionic.