Introductory Chemistry: A Foundation - Zumdahl S.S., DeCoste D.J. 2019
Energy
Thermochemistry (Enthalpy)
Objective
· To consider the heat (enthalpy) of chemical reactions.
We have seen that some reactions are exothermic (produce heat energy) and other reactions are endothermic (absorb heat energy). Chemists also like to know exactly how much energy is produced or absorbed by a given reaction. To make that process more convenient, we have invented a special energy function called enthalpy , which is designated by . For a reaction occurring under conditions of constant pressure, the change in enthalpy is equal to the energy that flows as heat. That is,
where the subscript “ ” indicates that the process has occurred under conditions of constant pressure and means “a change in.” Thus the enthalpy change for a reaction (that occurs at constant pressure) is the same as the heat for that reaction.
Interactive Example 10.5. Enthalpy
When mole of methane is burned at constant pressure, kJ of energy is released as heat. Calculate for a process in which a -g sample of methane is burned at constant pressure.
Solution
Where Are We Going?
We want to determine for the reaction of g of methane with oxygen at constant pressure.
What Do We Know?
· When mole of is burned, kJ of energy is released.
· We have g of .
What Do We Need to Know?
· Molar mass of methane, which we can get from the atomic masses of carbon ( g/mol) and hydrogen ( g/mol). The molar mass is g/mol.
How Do We Get There?
At constant pressure, kJ of energy per mole of is produced as heat:
Note that the minus sign indicates an exothermic process. In this case, a -g sample of is burned. Because this amount is smaller than mole, less than kJ will be released as heat. The actual value can be calculated as follows:
and
Thus, when a -g sample of is burned at constant pressure,
Reality Check The mass of methane burned is less than mole, so less than kJ will be released as heat. The answer has two significant figures as required by the given quantities.
Self-Check: Exercise 10.5
The reaction that occurs in the heat packs used to treat sports injuries is
· How much heat is released when g of is reacted with excess ?
See Problems 10.41 and 10.42.
Chemistry in Focus Burning Calories
There is a growing concern in the United States about the increasing tendency for individuals to be overweight. Estimates indicate that two-thirds of the adults in the United States are overweight, and one-third are classified as obese. This is an alarming situation because obesity is associated with many serious diseases such as diabetes and heart disease. In an effort to reduce this problem, the U.S. government now requires national restaurant chains and grocery stores to post the calorie counts for the food they sell. The hope is that people will use the information to make better food choices for weight control.
All of this leads to the question of how the calorie content of food is determined. The process involves a special type of calorimeter called a bomb calorimeter. The food sample is enclosed in the bomb calorimeter and burned. The energy produced heats the water surrounding the calorimeter, and the amount of energy is determined by measuring the increase in temperature of the known amount of water. The “Calorie” used for food is equal to the kilocalorie used by the science community. Thus the number of kilocalories produced by burning the food is the “Calorie” content of the food.
How is food “burned” in a calorimeter? The exact composition of food can be determined from its ingredients. Each ingredient in its proper amount for the food is burned in a calorimeter, and the calories released are determined. The calorie count assigned to a particular food is totaled as the sum of the ingredients and is adjusted for the amount of energy the body will actually absorb ( for fats and less for carbohydrates and proteins). Restaurants determine calorie counts from recipes. However, that means errors can occur, depending on how closely a chef follows a recipe and whether the portion size in the recipe is actually the size of the portion served in the restaurant. Although the “calorie” count may not be exact for restaurant foods, the listing on the menu will give people a chance to make better choices.
See Problem 10.43
Calorimetry
A calorimeter (Fig. 10.6) is a device used to determine the heat associated with a chemical reaction. The reaction is run in the calorimeter and the temperature change of the calorimeter is observed. Knowing the temperature change that occurs in the calorimeter and the heat capacity of the calorimeter enables us to calculate the heat energy released or absorbed by the reaction. Thus we can determine for the reaction.
Figure 10.6.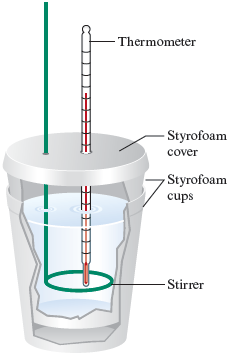
A coffee-cup calorimeter made of two Styrofoam cups.
Once we have measured the values for various reactions, we can use these data to calculate the values of other reactions. We will see how to carry out these calculations in the next section.