MCAT General Chemistry Review
Chapter 3: Bonding and Chemical Interactions
3.4 Intermolecular Forces
Atoms and compounds participate in weak electrostatic interactions. The strength of these intermolecular forces can impact certain physical properties, such as melting and boiling points. The weakest of the intermolecular interactions are the dispersion forces, also known as London forces. Next are the dipole–dipole interactions, which are of intermediate strength. Finally, we have the strongest type of interaction, the hydrogen bond, which is a misnomer because there is no actual sharing or transfer of electrons. We must keep in mind, however, that even hydrogen bonds, the strongest of these interactions, only have about 10 percent the strength of a covalent bond. Therefore, these electrostatic interactions can be overcome with small or moderate amounts of energy.
BRIDGE
These intermolecular forces are the binding forces that keep a substance together in its solid or liquid state and determine whether two substances are miscible or immiscible in solution. Solutions and solubility are discussed in Chapter 9 of MCAT General Chemistry Review.
LONDON DISPERSION FORCES
The bonding electrons in nonpolar covalent bonds may appear to be shared equally between two atoms, but at any point in time, they will be located randomly throughout the orbital. In a given moment, the electron density may be unequally distributed between the two atoms. This results in a rapid polarization and counterpolarization of the electron cloud and the formation of short-lived dipole moments. Subsequently, these dipoles interact with the electron clouds of neighboring compounds, inducing the formation of more dipoles. The momentarily negative end of one molecule will cause the closest region in any neighboring molecule to become temporarily positive itself. This causes the other end of the neighboring moleculeto become temporarily negative, which in turn induces other molecules to become temporarily polarized, and the cycle begins again. The attractive interactions of these short-lived and rapidly shifting dipoles are known as London dispersion forces, a type of van der Waals force.
Dispersion forces are the weakest of all of the intermolecular interactions because they are the result of induced dipoles that change and shift moment to moment. They do not extend over long distances and are, therefore, significant only when molecules are in close proximity. The strength of the London force also depends on the degree and ease by which the molecules can be polarized—that is, how easily the electrons can be shifted around. Large molecules with electrons that are far from the nucleus are easily polarizable and thus possess greater dispersion forces.
REAL WORLD
While dispersion forces (a type of van der Waals force) are the weakest of the intermolecular attractions, when there are millions of these interactions there is an amazing power of adhesion. This is demonstrated by geckos’ feet; the animal’s ability to climb smooth, vertical, and even inverted surfaces is due to dispersion forces.
Despite their weak nature, don’t underestimate the importance of dispersion forces. If it weren’t for them, the noble gases would not liquefy at any temperature because no other intermolecular forces exist between the noble gas atoms. The low temperatures at which noble gases liquefy are indicative of the very small magnitude of the dispersion forces between the atoms.
DIPOLE–DIPOLE INTERACTIONS
Polar molecules tend to orient themselves in such a way that the oppositely charged ends of the respective molecular dipoles are closest to each other: the positive region of one molecule is close to the negative region of another molecule. This arrangement is energetically favorable because an attractive electrostatic force is formed between the two molecules. This attractive force is denoted by dashed lines in most molecular notations and indicates a temporary bonding interaction, as shown in Figure 3.14.
 Figure 3.14. Dipole–Dipole Interactions in HCl
Figure 3.14. Dipole–Dipole Interactions in HCl
Dipole–dipole interactions are present in the solid and liquid phases but become negligible in the gas phase because of the significantly increased distance between gas particles. Polar species tend to have higher melting and boiling points than nonpolar species of comparable molecular weight due to these interactions. Realize that London forces and dipole–dipole interactions are different not in kind but in duration. Both are electrostatic forces between opposite partial charges; the difference is only in the transience or permanence of the molecular dipole.
BRIDGE
In organic chemistry, carbonyl groups possess distinct dipoles which facilitate nucleophilic attacks. This is the focus of almost all of the reactions in Chapters 6 to 9 of MCAT Organic Chemistry Review.
HYDROGEN BONDS
Hydrogen bonds are a favorite topic on the MCAT. A hydrogen bond is a specific, unusually strong form of dipole–dipole interaction, which may be intra- or intermolecular. Hydrogen bonds are not actually bonds—there is no sharing or transfer of electrons between two atoms. When hydrogen is bound to one of three highly electronegative atoms—nitrogen, oxygen, or fluorine—the hydrogen atom carries only a small amount of the electron density in the covalent bond.
The hydrogen atom essentially acts as a naked proton. The positively charged hydro-gen atom interacts with the partial negative charge of fluorine, oxygen, or nitrogen on nearby molecules. Substances that display hydrogen bonding tend to have unusually high boiling points compared to compounds of similar molecular weights that do not exhibit hydrogen bonding. The difference derives from the energy required to break the hydrogen bonds. Hydrogen bonding, shown in Figure 3.15, is particularly important in the behavior of water, alcohols, amines, and carboxylic acids.
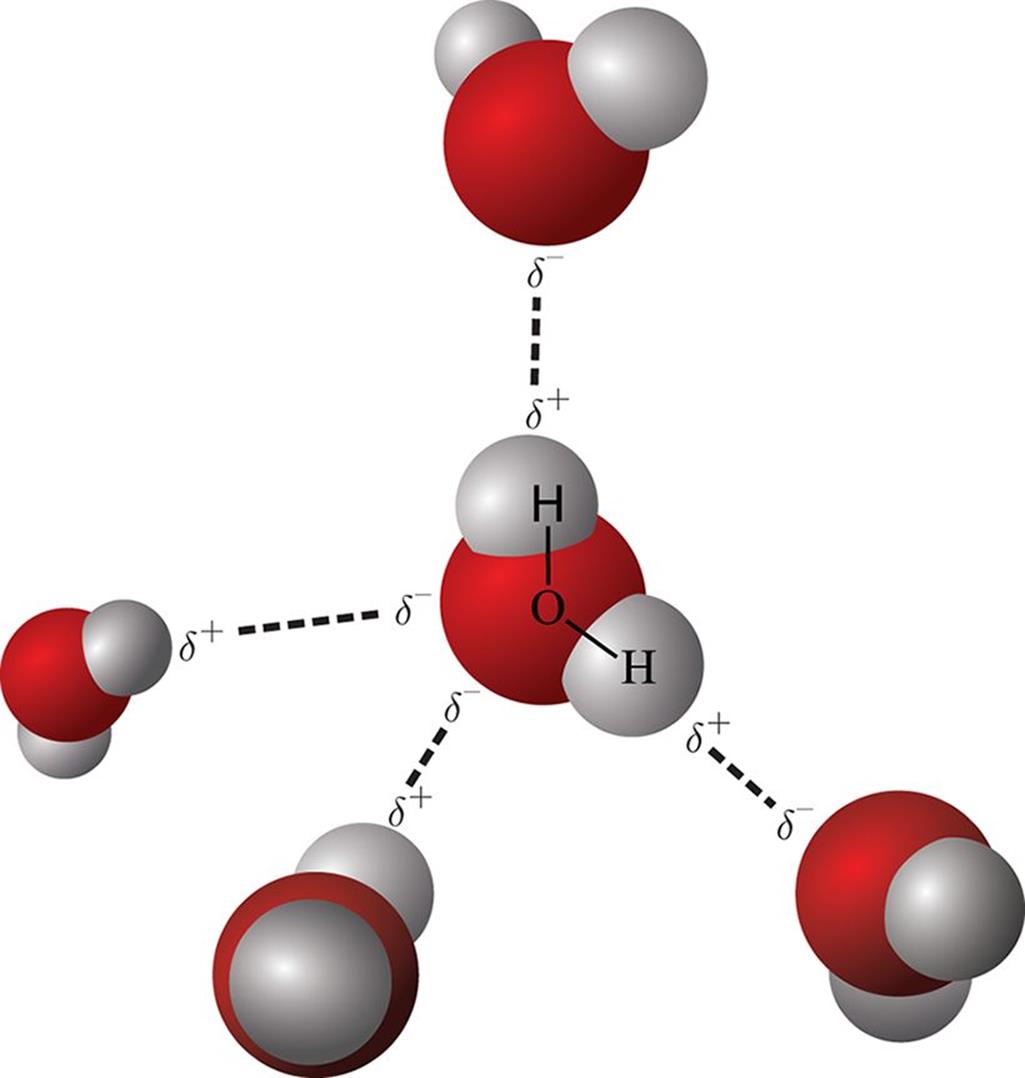 Figure 3.15. Hydrogen Bonding in Water
Figure 3.15. Hydrogen Bonding in Water
MNEMONIC
Hydrogen bonds: Pick up the FON (phone):
Hydrogen bonds exist in molecules containing a hydrogen bonded to Fluorine, Oxygen, or Nitrogen.
Many biochemical molecules, such as nucleotides, have different regions that are stabilized by hydrogen bonding, as shown in Figure 3.16. It is not an overstatement to say that—were it not for water’s ability to form hydrogen bonds and exist in the liquid state at room temperature—we would not exist (at least not in the form we recognize as “human”).
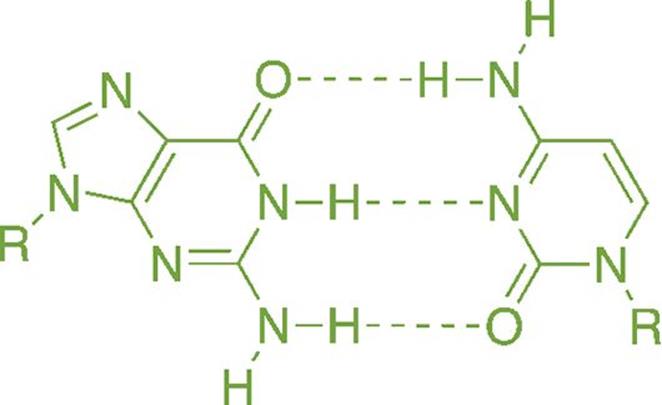 Figure 3.16. Hydrogen Bonding between Guanine and Cytosine
Figure 3.16. Hydrogen Bonding between Guanine and Cytosine
MCAT Concept Check 3.2:
Before you move on, assess your understanding of the material with these questions.
1. Rank the major intermolecular forces from strongest to weakest:
1.
2.
3.
2. Describe what occurs during dipole–dipole interactions.
3. In order to exhibit hydrogen bonding, what must be true of a given molecule?