The MCAT Chemistry Book - Aryangat A. 2012
Organic Chemistry
Phenols
A. INTRODUCTION
Phenol is a compound which has a hydroxyl group attached to a benzene ring. Although phenols have similar properties like alcohols (one aspect of similarity is that both phenols and general alcohols have the hydroxyl group), phenols are unique because of their aromatic ring.
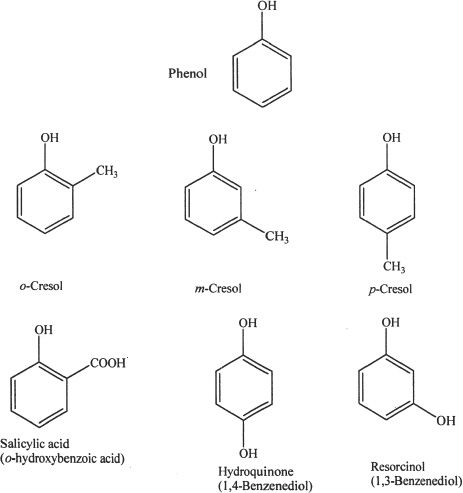
B. PROPERTIES OF PHENOLS
Hydrogen bonding is significant in phenols, because of the presence of the hydroxyl group. Phenols can form hydrogen bonds with other phenol molecules and water. Hence the boiling and melting points are relatively high for phenols. Phenols are acidic. In terms of acidity, phenols are in between carboxylic acids and alcohols.
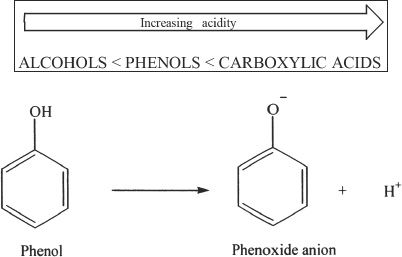
It is important to analyze the fact that the phenoxide ion’s negative charge is stabilized by the delocalization of the electrons into the benzene ring. This is one of the reasons for phenols’ increased acidity compared to alcohols. In alkoxide ions negative charge is localized in the oxygen atom (not delocalized).
C. EFFECTS OF SUBSTITUENTS ON THE ACIDITY OF PHENOLS
When a phenol has a strongly electron-withdrawing substituent group, the acidity of the phenol is increased. The reason for this increase is the electron-withdrawing group’s ability to delocalize the negative charge, more than the phenoxide ion itself.
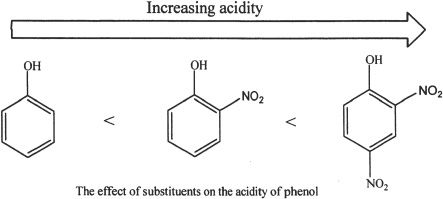
On the contrary, electron-donating substituents decrease the acidity of phenols.
D. REACTIONS OF PHENOLS
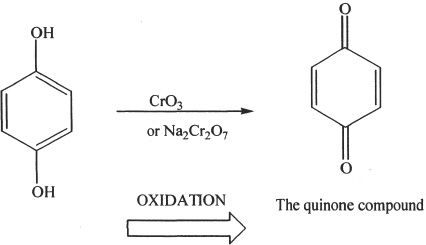
Formation of Quinones
Oxidation of phenols results in compounds called quinones. They are conjugated, and they contain two carbonyl groups (dicarbonyl). See the example below:
Sample reaction 26-1
E. ELECTROPHILIC AROMATIC SUBSTITUTION REACTIONS
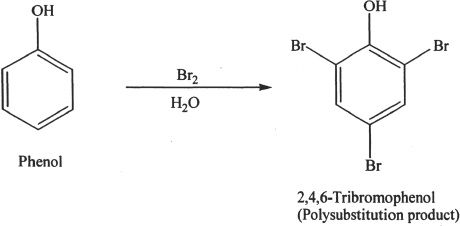
Certain compounds (electrophilic) can react with phenols (nucleophilic). The nucleophilic activity can be either in the aromatic ring or the oxygen in the hydroxyl group. Examples of electrophilic aromatic substitution include nitration, halogenation, Friedel-Crafts reactions (alkylation and acylation), and sulfonation. The halogenation example shown below is a polysubstitution reaction involving bromine. The polysubstitution usually occurs when polar solvents are used.
Sample reaction 26-2
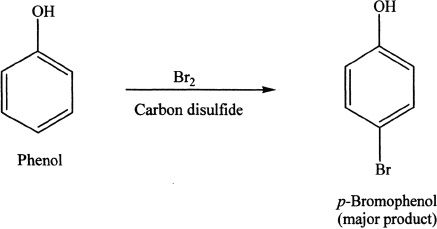
Monobromination can be achieved by using nonpolar solvents.
Sample reaction 26-3
CHAPTER 26 PRACTICE QUESTIONS
1. The product of the reaction sequence shown below is:
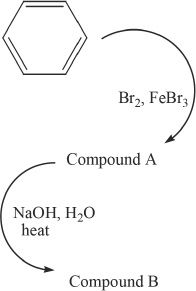
A. meta cresol.
B. phenol.
C. toluene.
D. styrene.
2. The reaction of phenol with Br2 / H2O can lead to all the following products in relatively significant quantities, except:
A. ortho-Bromophenol.
B. meta-Bromophenol.
C. 2,4,6-Tribromophenol.
D. all the above are formed in significant amounts.
3. Which of the following compounds can have significant amounts of intramolecular hydrogen bonding?
A. ortho-nitrophenol
B. para-nitrophenol
C. phenol
D. para-hydroxy benzaldehyde