The MCAT Chemistry Book - Aryangat A. 2012
Organic Chemistry
Amino Acids and Proteins
A. INTRODUCTION
Proteins are polymers of amino acids. Proteins are extremely important since they have a wide range of biological significance. They serve as nutrients, enzymes, cellular products of genes by translation (reflects the hereditary information), building material of muscles and other biologically important structures.
B. AMINO ACIDS
Amino acids are the monomeric units of proteins. The general structure of an amino acid is as follows:
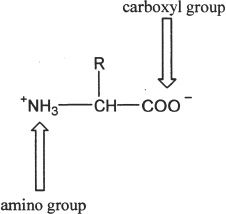
In the above diagram representing an amino acid, the characteristic amino and carboxyl groups are indicated by the arrows. Amino acids combine with other amino acids to form peptides.
Peptide Bond
An amido linkage formed between two amino acids is called peptide bond. Figure 28-1 shows the peptide bond between the amino acids, alanine and serine.
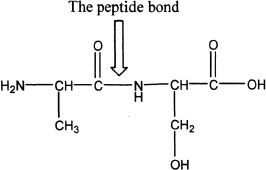
Figure 28-1
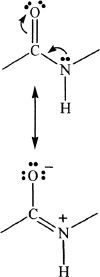
The four atoms present in a peptide bond can form two resonance structures.
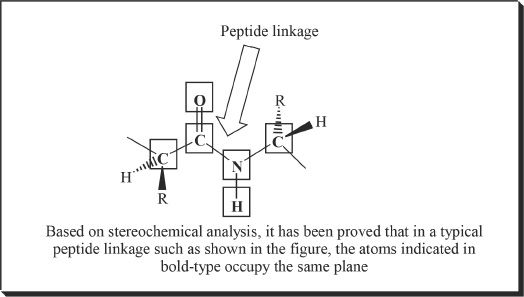
Figure 28-2
C. PEPTIDES
Dipeptide |
— contains two amino acids. |
|
Tripeptide |
— contains three amino acids. |
|
Tetrapeptide |
— contains four amino acids. |
|
Pentapeptide |
— contains five amino acids. |
|
Polypeptides |
— contain many amino acids. |
|
Proteins |
— contain fifty or more amino acids. |
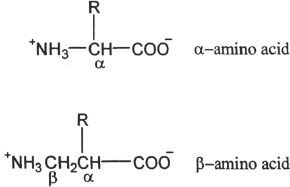
D. ALPHA AND BETA AMINO ACIDS
Amino acids can be classified as α, β, γ, and so on. If the amino group is attached to the carbon next to the carboxylic carbon, the amino acid is an α—amino acid. If it is attached to carbon next to the α—carbon, the amino acid is a β—amino acid.
E. DIPOLAR NATURE OF AMINO ACIDS
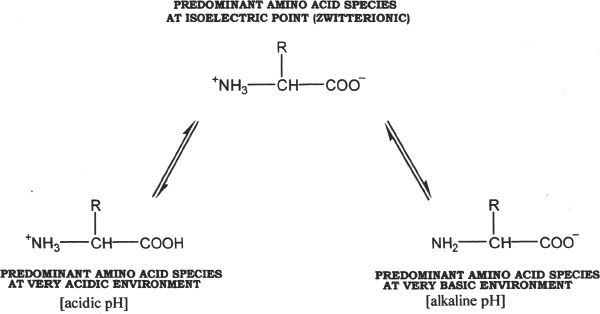
Amino acids possess a dipolar nature. They are amphoteric, since they have both acidic and basic functional groups. Dipolar ions are often referred as zwitterions. In certain pH ranges, the amino acids exist predominantly as zwitterions, with a net charge of zero.
F. CLASSIFICATION OF AMINO ACIDS
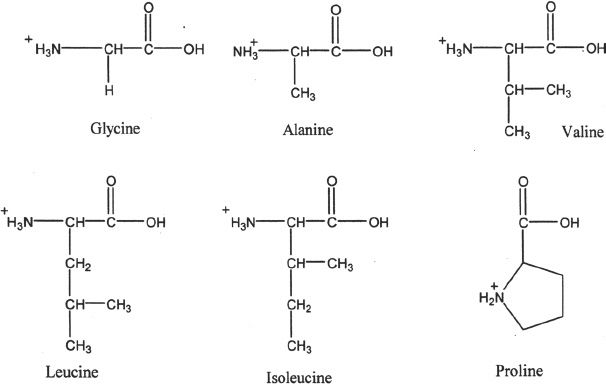
On the basis of the R groups present in the amino acids, they can be classified into various groups. There are about 20 important amino acids that are found in proteins.
HYDROPHIC AMINO ACIDS
1. Nonpolar amino acids with aliphatic R groups
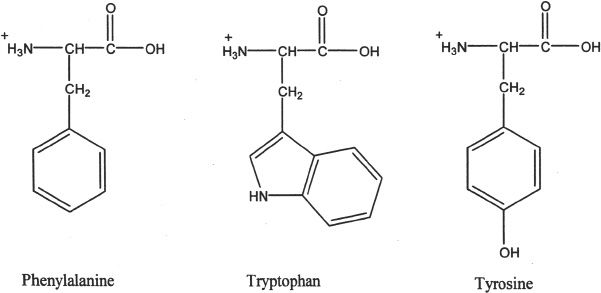
2. Nonpolar amino acids with aromatic R groups
HYDROPHILIC AMINO ACIDS
1. Polar amino acids
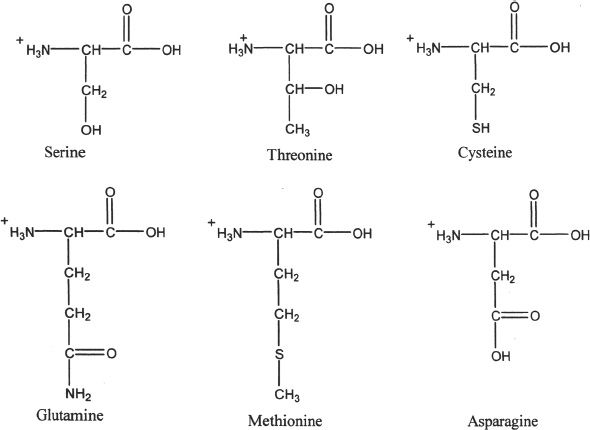
2. Acidic amino acids
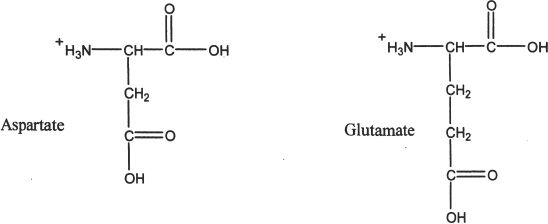
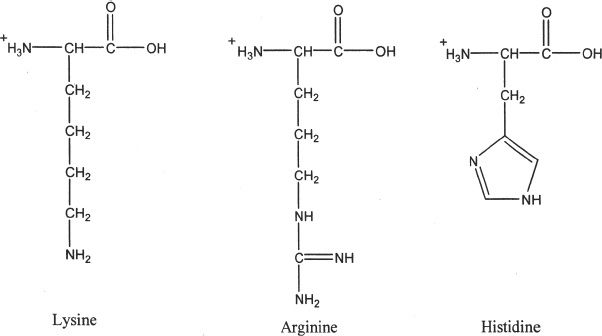
HYDROPHILIC AMINO ACIDS
3. Basic amino acids
G. PROPERTIES OF AMINO ACIDS
Amino acids have ionizable groups. Each ionizable group has its own pKa value, which is the pH at which half of that particular ionizable group is in the dissociated state and the other half in the undissociated state. For the MCAT you should be familiar with the concept of isoelectric point. Isoelectric point of an amino acid is the pH at which the net charge of the amino acid is zero. Amino acids can sometimes act as acids or bases. This nature (property) of amino acids is called amphoteric property.
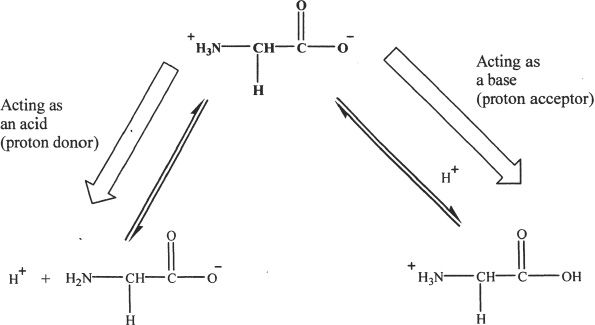
Figure 28-3
The amino acid shown in the above example is glycine. The amphoteric species is shown on top (bold faced).
H. PROTEINS
The structure of proteins can be classified into primary, secondary, tertiary, and quaternary structures, according to the level of complexity of the proteins.
Primary Structure of Proteins
The simple sequence of amino acids in a protein is called the primary structure of the protein. These sequences of amino acid chains can bend, fold, and bond to form more complex structures of proteins, which can be described in terms of secondary, tertiary, and quaternary structures. Figure 28-4 shows a tetrapeptide with amino acids: alanine, lysine, glycine, and phenylalanine.
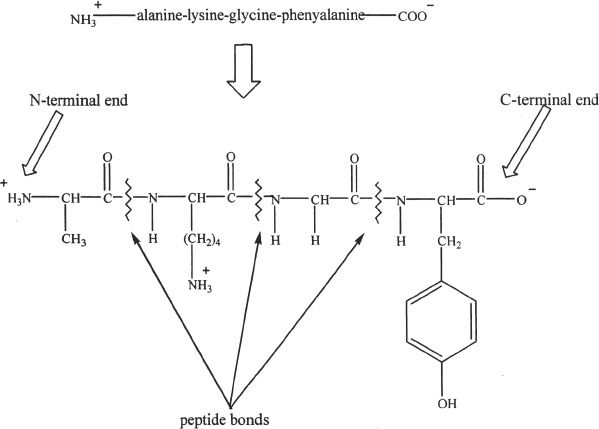
Figure 28-4
A peptide chain has an amino acid with the free (without a peptide bond) amino group on one end (the N-terminal end), and an amino acid with free (without a peptide bond) carboxylic group on the other end (the C-terminal end).
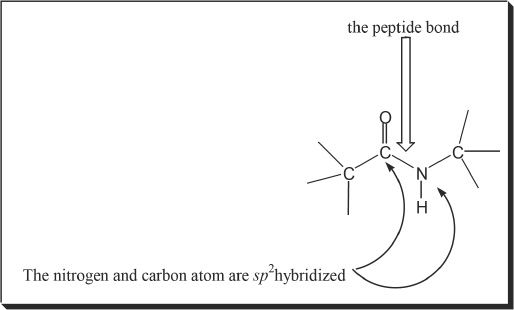
Figure 28-5
Secondary Structure of Proteins
The two main types of secondary structural arrangements are the α-helix and the β-pleated sheet. Generally, these conformations generate the secondary structure of proteins.
1. α-helix
The α-helical structure or conformation is one of most important aspects of the secondary structure of proteins. This conformation of peptide chain has interactions within its chains by hydrogen bonding. A simple arrangement can be described as a helical structure in which the long polypeptide chain is wound around the long axis. The side chains (R groups) of the amino acids extend out of the helical backbone. Figure 28-6 shows right- and left-handed α-helical structures.
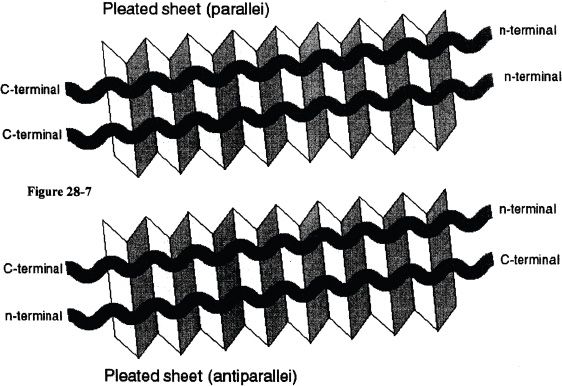
2. β-pleated sheet
These represent the sheetlike arrangement of the polypeptide chains. The hydrogen bonds are found between the adjacent polypeptide chains. The polypeptide chains involved in the pleated sheet structure can be either parallel or antiparallel. Hydrogen bonds stabilize the β-pleated sheet (see Figure 28-7).
Tertiary Structure of Proteins
The tertiary structure of proteins is the three dimensional arrangement of the polypeptide chain. Tertiary structure depicts the way in which the secondary structure folds to form the three dimensional form. Different kinds of bonds or interactions are responsible for the maintenance of the tertiary structure. They include hydrophobic forces, hydrogen bonds, disulfide bonds, salt bridges, and Van der Waal forces.
Disulfide bonds: These are bonds formed by two cysteines.
Hydrophobic bonding: Hydrophobic bonding or forces are weak interactions between hydrophobic side chains of the hydrophobic amino acids in the protein.
Quaternary Structure of Proteins
Some proteins can be complex, when they contain multiple subunits of polypeptide structural entities. The way in which three-dimensional subunits interact to form the complete functional protein is called the quaternary structure of a protein. This level of hierarchy is possible only if the protein has multiple units. An example is hemoglobin.
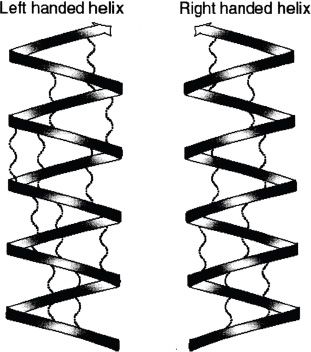
Figure 28-6
I. DENATURATION OF PROTEINS
Proteins can be denatured in many ways. A common example that we all have seen is the denaturation of egg albumin when we cook them. In this case, the denaturing agent is the temperature. Most biologically significant proteins are extremely heat sensitive. An increase in temperature can disrupt hydrogen bonds as well as hydrophobic interactions in proteins. This is one reason why we cannot survive in extreme (hot or cold) temperatures because at extreme temperatures, the proteins and other sensitive chemicals in our body denature and cease to function. Besides temperature changes, pH changes can also affect proteins. Other denaturing agents include detergents, oxidizing agents, reducing agents, UV light, and pressure.
Generally, protein denaturation includes the complete or partial unfolding of the polypeptide chain, cleavage of disulfide linkages, and breakage of noncovalent interactions. Denaturation is sometimes reversible. The reversing process is called renaturation.
CHAPTER 28 PRACTICE QUESTIONS
1. Which of the following is a basic amino acid?
A. Valine
B. Tryptophan
C. Arginine
D. Threonine
2. All the following amino acids are optically active, except:
A. Valine
B. Proline
C. Glutamic acid
D. Glycine
3. At a particular pH, the amino acid glutamic acid has a net charge of zero. When this pH is achieved, it is said to be at glutamic acid’s:
A. isobaric point.
B. isomeric point.
C. isotonic point.
D. isoelectric point.
4. Which of following groups are not present in any of the common amino acids?
A. Amino group
B. Carbonyl group
C. Aldehyde group
D. All the above groups are present.
5. Amino acids can be bonded to each other covalently by means of:
A. hydrogen bonds.
B. dipole-dipole interactions.
C. phosphodiester linkages.
D. peptide linkages.
6. Proteins are made of:
A. monosaccharides.
B. sugars.
C. lipids.
D. amino acids.
7. Which of the following will certainly not affect the structure of a protein?
A. Proteases
B. Strong bases
C. Strong acids
D. All the above can have some effect on the structure of proteins.
8. Which amino acid is responsible for the formation of disulfide bonds?
A. lysine
B. phenylalanine
C. tryptophan
D. cysteine
Questions 9-14 are based on the following passage.
Passage 1
Amino acids play an essential role in the physiological processes in our body. They are the main building blocks of the principal structures in our body. Amino acids themselves or in combination with carbohydrates, lipids, etc., form an intricate system of physiologically important structures and functional domains without which life as we know it cannot exist.
From a biological point of view with respect to life forms, only 20 amino acids are important, even though many more are found in nature. Most amino acids are optically active. With the functional groups and their chemical behavior, we can predict certain properties of amino acids such as ionization properties. Amino acids join together to form peptides. Table-1 shows the pKa values of some amino acids.
Table-1
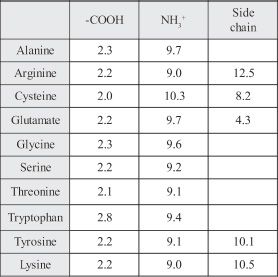
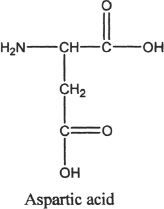
9. Which of the following are true regarding amino acids?
I. Amino acids have carboxyl group.
II. Amino acids have high melting points.
III. Some amino acids can have a net positive charge.
IV. Some amino acids can have a net negative charge.
A. I, III & IV only
B. I, II & III only
C. I, III & IV only
D. I, II, III & IV
10. Which of the following represents the pH at which there is no net movement of the amino acid when subjected to an electric field?
A. pH at pKa
B. pH at pKb
C. pH at isoelectric point
D. There is no such pH for amino acids because some amino acids are acidic, some are basic, and some are neutral.
11. Which of the following represents a peptide bond?
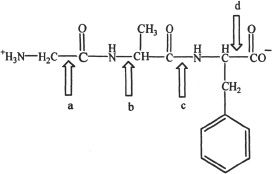
A. a
B. b
C. c
D. d
12. In our body, some amino acids are changed into metabolic intermediates which can enter metabolic cycles. One such example is given below. The example shows the conversion of serine into pyruvate which can be converted to acetyl CoA and enter the Krebs cycle. Compound Y can be best described as:
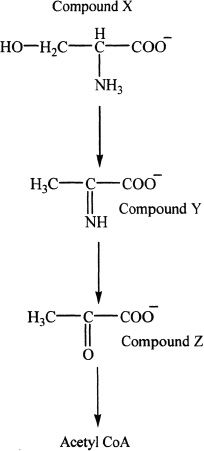
A. an amide.
B. an imine.
C. a phenol.
D. an imidazol.
13. Which of the following amino acids will migrate toward the positive electrode when subjected to an electric field at a pH of 7.0?
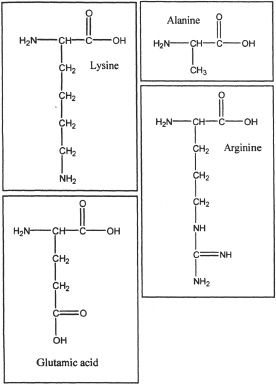
A. Alanine
B. Arginine
C. Lysine
D. Glutamic acid
14. All the following are not basic amino acids, except:
A. Glutamate.
B. Aspartate.
C. Alanine.
D. Lysine.