The MCAT Chemistry Book - Aryangat A. 2012
Organic Chemistry
Carbohydrates
A. INTRODUCTION
Carbohydrates, commonly known as sugars, are the most abundant group of biomolecules in nature. Our daily regimen of foods includes different forms of carbohydrates. Potatoes, rice, bread, pasta are carbohydrate-rich sources of food. There are different types of carbohydrates. The simplest units are called monosaccharides. A disaccharide contains two monosaccharides. A polysaccharide contains many monosaccharide subunits.
B. MONOSACCHARIDES
Based on the number of carbons, monosaccharides can be called trioses (three carbons), tetroses (four carbons), pentoses (five carbons), hexoses (six carbons), heptoses (seven carbons), octoses (eight carbons), and so on.
Monosaccharides contain many hydroxyl groups and a carbonyl group. If a monosaccharide contains an aldehyde group, it is called an aldose sugar. If a monosaccharide contains an keto group, it is called an ketose sugar.
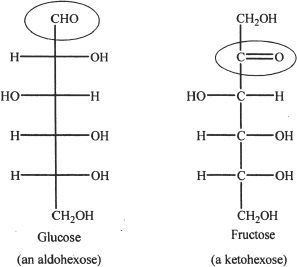
Figure 29-1 Glucose is an aldose sugar. Fructose is a ketose sugar.
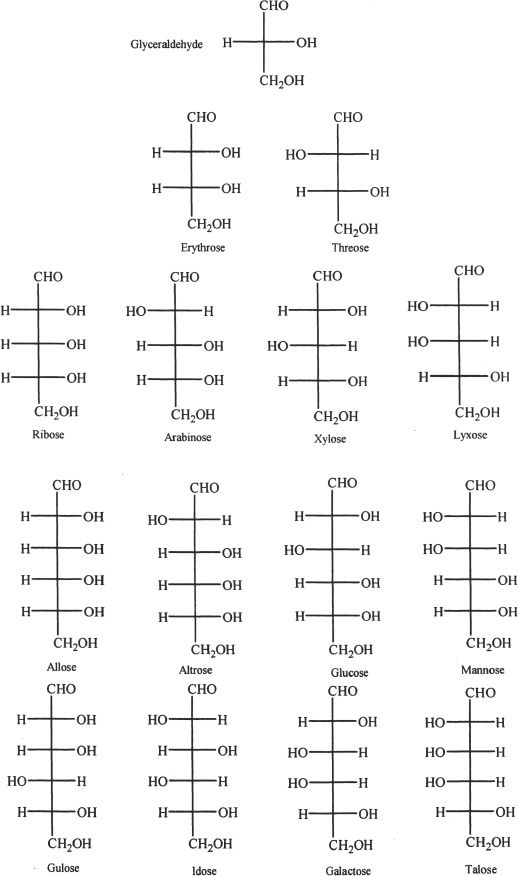
Figure 29-2 D-aldose sugars
C. CONFIGURATIONS OF MONOSACCHARIDES
Monosaccharides have chiral centers and thus exhibit optical activity. Let’s explore the structure of glyceraldehyde. In the three-dimensional representation, the dotted wedges indicate the bonds that extent backwards from the chiral carbon (away from you or into the plane of the page); and the solid wedges indicate the bonds that are projected toward you (out of the plane of the page). In stereochemistry, Fischer projection is an important way to represent the spatial orientation of molecules. In Fischer projection representation, the bonds that are pointed backward (away from you or into the page) are indicated by vertical lines, and the bonds that extend toward you (out of the page) are represented by horizontal lines. For a more detailed approach, refer to Chapter 19 (Stereochemistry).
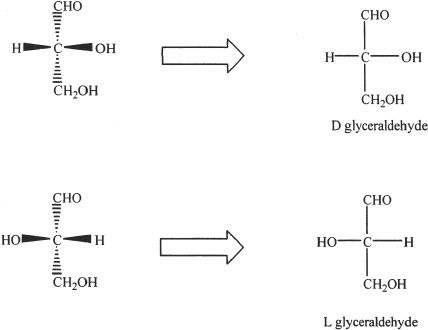
Figure 29-3
By convention, the carbonyl group is always drawn on top. With that as the standard, we can describe the structure of D-glyceraldehyde as follows:
D-glyceraldehyde has a hydroxyl group represented on the right side of the chiral carbon. L-Glyceraldehyde has a hydroxyl group represented on the left side of the chiral carbon. To determine whether a sugar structure shown is L or D, we have to locate the chiral carbon that is farthest from the carbonyl group, and see whether the hydroxyl group is on the right or on the left of the chiral carbon.
D. CYCLIC STRUCTURE OF HEXOSES
Glucose
Glucose is a six-carbon aldohexose. The straight chain hexose structures can become cyclic. When aldehydes and ketones undergo reactions with alcohols, hemiacetals or hemiketals are formed. In aldohexoses, the cyclic structure is formed when the hydroxyl group in the fifth carbon reacts (nucleophilic addition) with the carbonyl carbon of the aldehyde group. The product formed is a hemiacetal. The cyclization is represented in Figure 29-4.
A few facts worth remembering
1) The six-membered (hemiacetal) ring formed is called the pyranose ring.
2) The oxygen in the ring is from the hydroxyl group involved in the ring closure.
3) The carbon which is denoted as “carbon one” in the cyclic structure is the carbonyl carbon of the straight chain (the aldehyde group).
4) Notice that the cyclic structure can go either way in the sense that the resulting cyclic structure can either be an α- or a β-anomer.
5) The carbon number `one’ shown in the cyclic structure is called the anomeric carbon.
6) In aqueous solutions, the α- and β-anomers can interconvert, and the process is termed mutarotation.
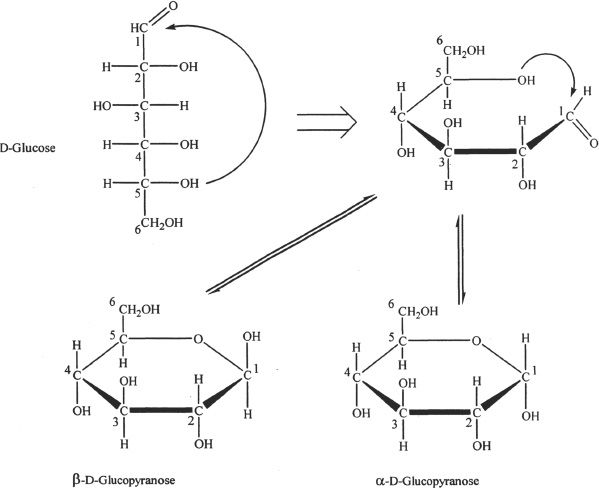
Figure 29-4 The formation of cyclic structures of glucose
Fructose
Fructose is a ketohexose sugar. The cyclic structure formed is called a hemiketal, and the five membered ring that is formed is called furanose ring. The straight chain structure and the cyclic structures of fructose are shown in Figure 29-5.
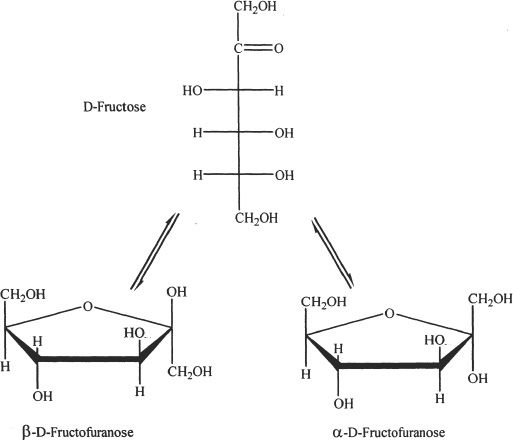
Figure 29-5
Epimers are any two isomeric sugars which differ in their configurations only at one of the chiral carbon atoms. Glucose and mannose are epimers. Glucose and galactose are also epimers.
E. OXIDATION OF MONOSACCHARIDES
Monosaccharides can be oxidized. Benedict’s reagent (oxidizing agent) can accomplish this by reacting with the aldehyde group of the open-chain form. Thus aldose and ketose sugars are reducing sugars. In fact, all monosaccharides are reducing sugars. Some disaccharides can be oxidized by Benedict’s reagent.
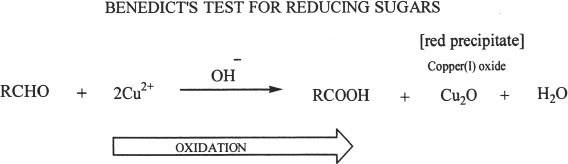
Benedict’s reagent is reduced in the process to cuprous oxide.
F. DISACCHARIDES
Disaccharides are sugar units containing two monosaccharides linked by a covalent bond called glycosidic bond. Maltose, sucrose, and lactose are examples of disaccharides. Maltose is formed by the linkage of two glucose molecules by a glycosidic bond. Sucrose is a combination of glucose and fructose. Lactose is composed of glucose and galactose. The hydrolysis of disaccharides gives their corresponding monosaccharide subunits.
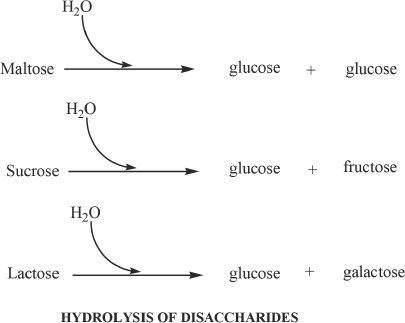
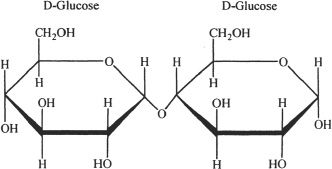
Figure 29-6 The structure of maltose
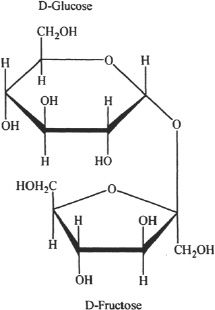
Figure 29-7 The structure of sucrose
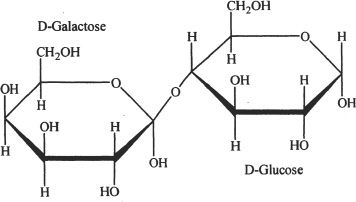
Figure 29-8 The structure of lactose
G. POLYSACCHARIDES
Polysaccharides are polymers of monosaccharides. Starch, glycogen, and cellulose are examples of polysaccharides. Polysaccharides can be homogeneous in terms of its fundamental unit (monosaccharide). If more than one type of monosaccharide is present in a polysaccharide, then it is called a heteropolysaccharide.
Starch
Starch is composed of two types of polymer structures derived from α-glucose - a linear polymer form called amylose, and a branched polymer form called amylopectin. Figure 29-9 shows the two types of glucose polymers involved in the formation of starch. The amylose has α(1->4) linkages of glucose subunits, and the amylopectin has α(1->6) and α(1->4) linkages.
Glycogen
Glycogen is a polysaccharide found in most animals as a storage medium of carbohydrate. It is stored mainly in the skeletal muscles and the liver. We can say that starch is to plants as glycogen is to animals. In other words, the animal carbohydrate storage medium is glycogen, and the plant carbohydrate storage medium is starch. The structure of glycogen is mostly like that of the amylopectin structures found in starch, only with more branching.
Cellulose
Cellulose is a polysaccharide found in plants. But unlike starch and glycogen, the monosaccharide units that build up the cellulose are β-glucose units. Also the polysaccharide chains found in the cellulose structure are unbranched. In addition, the linkages in cellulose units are β-linkages instead of β-linkages. This type of β-linkage is the reason why humans cannot digest cellulose. To be more precise, the enzymes that we have do not work on such linkages. Some herbivores such as cattle have cellulose digesting bacteria in their stomachs which digest the foods containing cellulose. Once the bacterial enzymes break the cellulose structures into simple sugars, then the animal can use them for its metabolic needs.
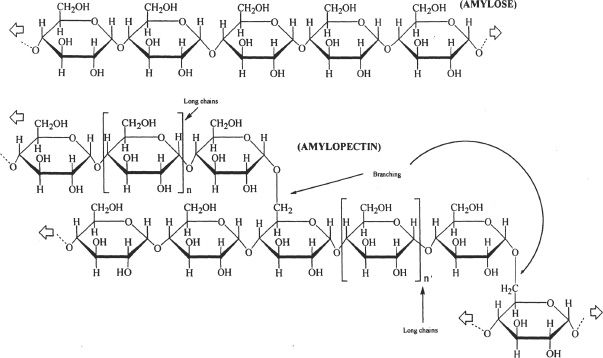
Figure 29-9 The glucose polymers that are involved in the formation of starch
CHAPTER 29 PRACTICE QUESTIONS
1. All the following are monosaccharides, except:
A. galactose
B. glucose
C. ribose
D. maltose
2. What is the name of the structure drawn below?
A. D-glucose
B. L-mannose
C. D-galactose
D. L-ribose
3. A lab technician making a fresh sugar solution noticed that the optical activity of the sugar solution changed after the solution had been standing for several hours. This change in optical activity is best explained by the phenomenon called:
A. Tyndall effect.
B. Brownian movement.
C. optical anomaly.
D. mutarotation.
4. Which of the following is NOT a disaccharide?
A. Maltose
B. Sucrose
C. Glycogen
D. Lactose
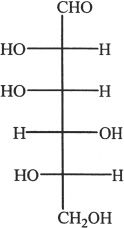
5. The structure shown below is that of a monosaccharide. How many optical isomers are possible for this structure?
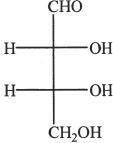
A. 2
B. 4
C. 6
D. 9
Questions 6-11 are based on the following passage.
Passage 1
The structures of some monosaccharides are drawn below.
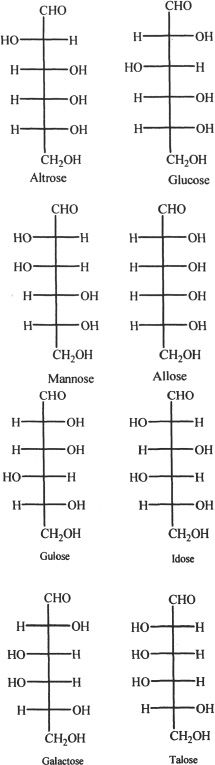
6. All the following are monosaccharides, except:
A. sucrose.
B. fructose.
C. glucose.
D. galactose.
7. The structure given below is:
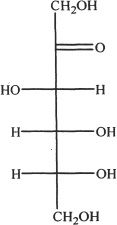
A. glucose.
B. ribose.
C. fructose.
D. maltose.
8. Which of the following statements are true regarding the two structures (Compounds I & II) drawn below?
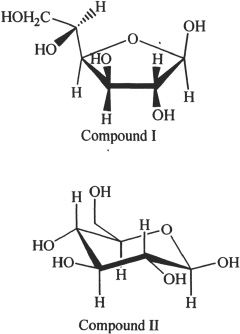
Statement (1) - Compound I is glucose.
Statement (2) - Compound II is glucose.
Statement (3) - Compound II is fructose.
Statement (4) - Compound II is fructose.
A. Statements 1 & 2
B. Statements 2 & 3
C. Statements 3 & 4
D. Statements 2 & 3
9. Glucose and mannose are:
A. anomers.
B. epimers.
C. enantiomers.
D. non superimposable mirror images.
10. The structure of a disaccharide is shown below. The bond indicated by the arrow is called:
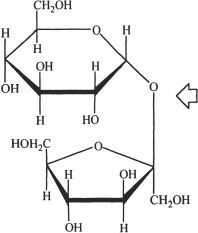
A. a glycosidic linkage.
B. a peptide linkage.
C. a glucose linkage.
D. an ester linkage.
11. The two stereogenic structures shown below are best related by the term:
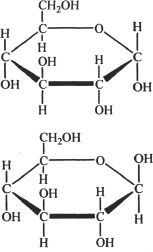
A. enantiomers.
B. resonance structures.
C. anomers.
D. mesomers.