The MCAT Chemistry Book - Aryangat A. 2012
Organic Chemistry
Lipids
A. INTRODUCTION
Lipids are a group of compounds which are hydrophobic, and have nonpolar groups predominantly. They can also contain polar groups. Lipids are important to living beings. Fats, a form of lipid, are used to store energy in organisms. Lipids are found in a wide variety of capacities; as hormones, as part of membranes, and as cofactors. Lipids are water insoluble. This makes them different and unique for their roles as biomolecules. Examples of lipids include waxes, steroids, and prostaglandins.
B. FATTY ACIDS
Fatty acids are carboxylic acids with long hydrocarbon chains. If the hydrocarbon chain in a fatty acid is saturated (no carbon-carbon double bonds), then it is called a saturated fatty acid. If there are (one or more) carbon-carbon double bonds in the hydrocarbon chain of a fatty acid, then it is called an unsaturated fatty acid. As the length of the hydrocarbon chain increases, the lipid solubility of fatty acids increases, and the water solubility decreases. Fats and oils are derivatives of fatty acids.
Table 30-1
|
Saturated Fatty Acids |
|
CH3(CH2)12COOH |
Myristic acid |
CH3(CH2)14COOH |
Palmitic acid |
CH3(CH2)16COOH |
Stearic acid |
Table 30-2
|
Unsaturated Fatty Acids |
||
H3C(H2C)7HC=CH(CH2)7COOH |
Oleic acid |
|
H3C(H2C)4HC=CHCH2CH=CH(CH2)7COOH |
Linoleic acid |
|
H3CH2CHC=CHCH2CH=CHCH2CH=CH(CH2)7COOH |
Linolenic acid |
|
C. TRIACYLGLYCEROLS
Triacylglycerols are esters of fatty acids. Fats or triglycerides are triacylglycerols. In a triacylglycerol, a glycerol molecule is linked with three fatty acids by ester linkages. The structure of glycerol is shown below:

The fatty acids that are present in fats are mostly straight-chain fatty acids (saturated and unsaturated), with even number of carbon atoms. According to the number of fatty acids esterifying the glycerol’s hydroxyl groups, the glyceride formed could be a monoglyceride, a diglyceride or a triglyceride. Glycerides with long chain fatty acids are insoluble in water.
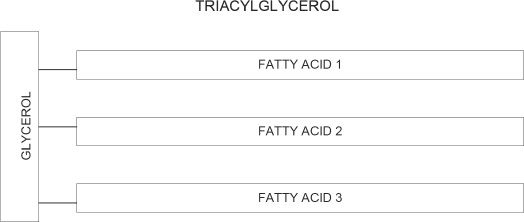
Figure 30-1
A diagrammatic representation of a triglyceride (triacylglycerol) is shown in Figure 30-1. In the figure, the vertical bar represents glycerol and the horizontal bars represent the fatty acids. The fatty acids (1, 2, & 3) present in a triacylglycerol molecule can be the same three fatty acids, or can be different fatty acids.
Fats can be solid or liquid. This physical property is a direct effect of the fatty acid substituents that are present in them. When fats are in their liquid state they are commonly called oils. Broadly we can generalize that solid fats mainly contain saturated fatty acids, and liquid fats mainly contain unsaturated fatty acids. We should not confuse this generalization with the fact that the naturally occurring fats and oils contain many different types of fatty acids, both saturated and unsaturated. The physical properties of fats are mostly a function of the fatty acids that are present in them. For example, human stored fat contains predominantly oleic acid (a saturated fatty acid) which constitutes about 47 % of the total fatty acid content. It also contains palmitic acid, linoleic acid, stearic acid, myristic acid, and other fatty acids in decreasing amounts respectively.
D. STEROIDS
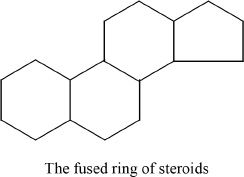
A steroid has a drastically different structure from that of fats. Steroids are lipids having a particular fused-ring structure; a ring structure which has three six-carbon rings and a five-carbon ring. All steroids have this basic unit in their structures. Steroids include a wide variety of molecules, including cholesterol and its derivatives such as steroid hormones, and have tremendous biological significance. The steroid hormones include adrenocorticoid hormones and sex hormones.
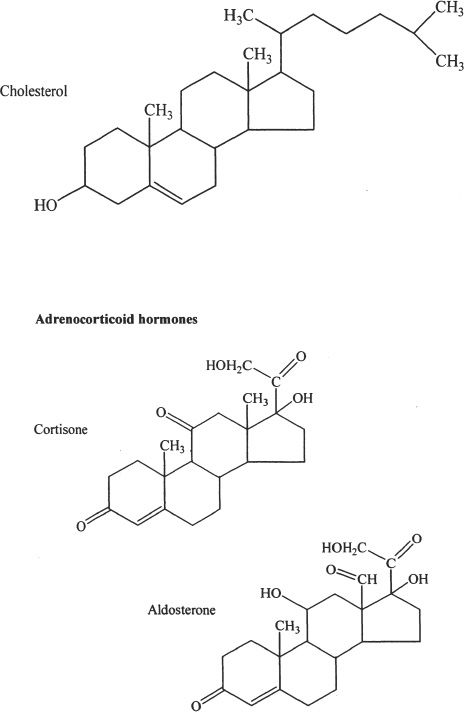
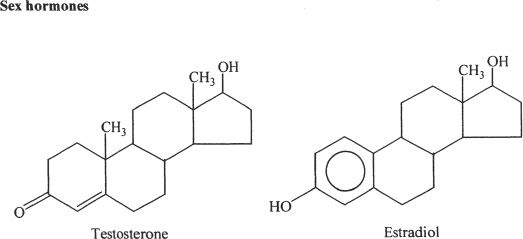
Notice that all these compounds have the fused-ring structure that we mentioned earlier. In fact, cholesterol acts as the starting material for the synthesis of steroid hormones. The steroid hormones play a very important role in the proper development and functioning of our body. The structures given above are just for reference and understanding the general structural integrity of steroids. You don’t have to memorize them.
CHAPTER 30 PRACTICE QUESTIONS
1. If 1 mol of triglyceride is subjected to complete hydrolysis by using a lipase enzyme, the products formed are:
A. 3 moles of glycerol and 1 mol of fatty acid.
B. 1 mol of glycerol and 3 moles of fatty acid.
C. 1 mol of glycerol and 1 mol of fatty acid.
D. 2 moles of cholesterol.
2. Which of the following is not a steroid?
A. Vitamin D
B. Testosterone
C. Estradiol
D. All the above are steroids.