The MCAT Chemistry Book - Aryangat A. 2012
Organic Chemistry
Phosphoric Acid Chemistry
A. INTRODUCTION
Phosphoric acid is H3PO4. It is an important molecule with respect to its part in a wide variety of biomolecules in our body such as adenosine triphosphate (ATP), deoxyribonucleic acid (DNA), and ribonucleic acid (RNA).
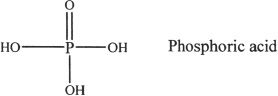
B. DISSOCIATION OF PHOSPHORIC ACID
Phosphoric acid is a triprotic acid. That means it has three pKa values.
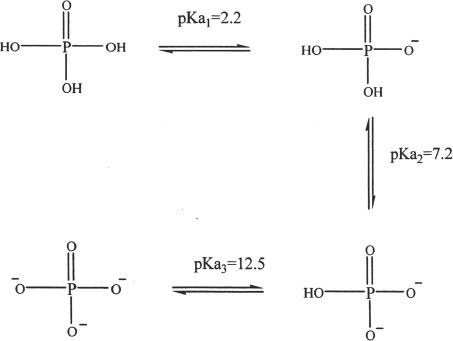
C. ESTERS AND ANHYDRIDES
Phosphoric acid can form esters with alcohols. It can form monoesters, diesters, and triesters. A general representation of the formation of phosphoesters is given below. R represents the alkyl group.
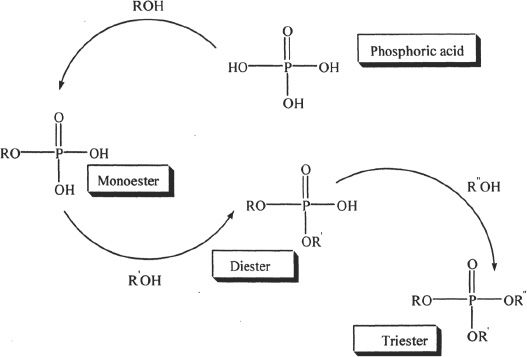
Figure 31-1
Besides esters, phosphoric acid forms anhydrides such as phosphoric anhydrides by reacting with themselves. One such combination is shown in Figure 31-2.
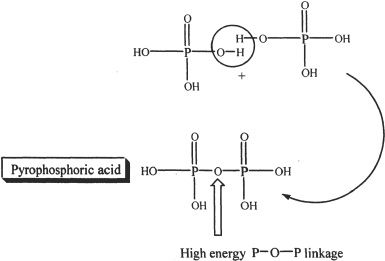
Figure 31-2
These anhydrides are biologically useful since the —P—O—P— linkages found in them are high energy linkages, and are used by the body to store energy. When energy is required, the linkage is hydrolyzed to give off the stored energy which is in turn used for the metabolic processes in the body.
D. BIOLOGICAL SIGNIFICANCE OF PHOSPHORIC ACID
We cannot completely fathom the importance of phosphoric acid in the biological processes. It is a part of our genetic make up in the sense that phosphoric acid is a part of the deoxyribonucleic acid (DNA) and the ribonucleic acid (RNA). The deoxyribose sugars in the DNA are connected using phosphoric acid entities by forming linkages called phosphodiester bonds. These phosphodiester bonds connect the 3’ hydroxyl group of one sugar to the 5’ hydroxyl group of the next sugar forming the backbone of the nucleic acids. See Figure 31-3.
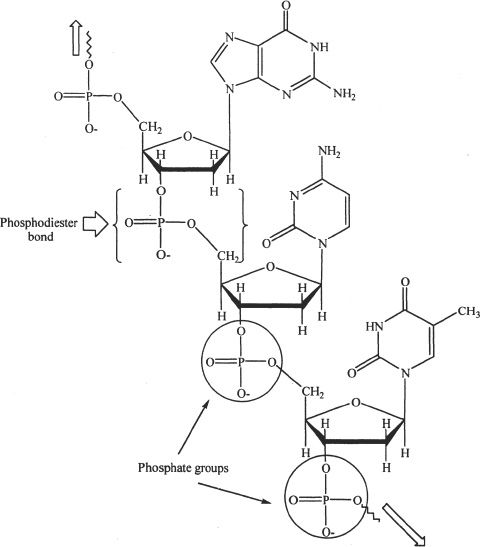
Figure 31-3 Part of a DNA backbone showing the phosphodiester bonds. The deoxyribose sugars are linked by the phosphate groups (indicated by the circles).
At normal biological pH range (7.35-7.45), the phosphate esters usually show a double negative charge. This property of the phosphate esters makes them generally water soluble, and thus perfect for the physiological environment in our body.
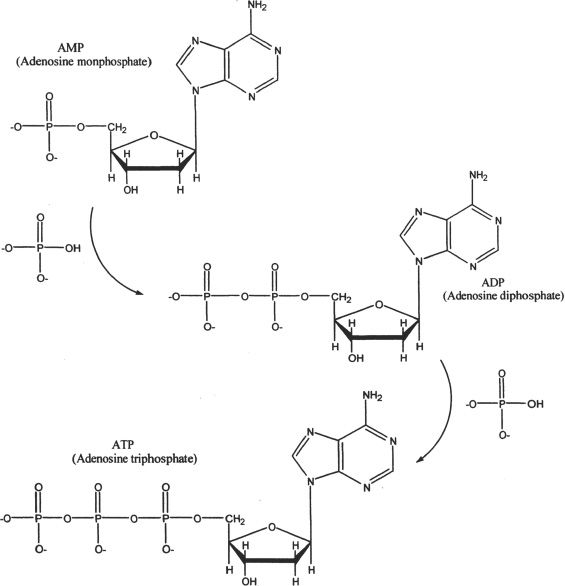
Another noteworthy biomolecule that contains the phosphate groups is the ATP (adenosine triphosphate). The ATP molecules act as one of the major energy currencies of living beings. It is a triphosphate ester. The formation of ATP from AMP (adenosine monophosphate) is shown in Figure 31-4.
Figure 31-4 The formation of ATP from AMP
CHAPTER 30 PRACTICE QUESTIONS
Questions 1-7 are based on the following passage.
Passage 1
Phosphoric acid is an integral part of some of the most important biomolecules in our body.
Reaction 1
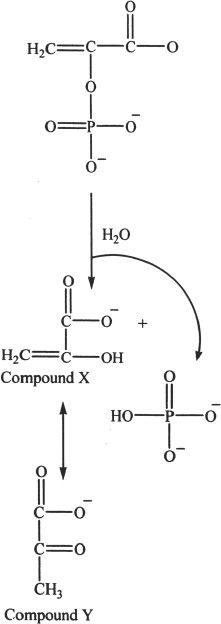
Reaction 1 depicts the conversion of phosphoenol pyruvate to pyruvate. Phosphate is a part of many high-energy compounds in biological systems. Examples include ATP (adenosine triphosphate)) and GTP (guanosine triphosphate).
1. The bond indicated by the arrow is a:
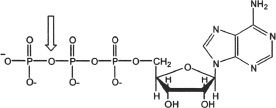
A. phosphate ketone bond.
B. phosphate anhydride bond.
C. phosphate ester bond.
D. N-glycosidic bond.
2. Reaction 1 can be best described as a:
A. reduction reaction.
B. dehydration reaction.
C. dehydrohalogenation reaction.
D. hydrolysis reaction.
3. The interconversion of Compounds X and Y is called:
A. resolution.
B. tautomerism.
C. Tyndall effect.
D. Brownian movement.
4. The bond indicated by the arrow is a:
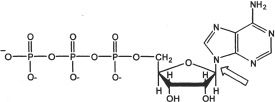
A. phosphate ketone bond.
B. phosphate anhydride bond.
C. phosphate ester bond.
D. N-glycosidic bond.
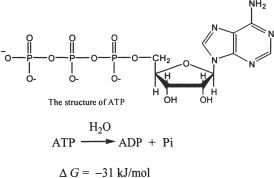
5. The phosphate anhydride bonds present in the ATP molecules are high-energy bonds. Which of the following is true regarding the hydrolysis of these bonds?
A. The products of hydrolysis have less energy than that of the reactants.
B. The products of hydrolysis have the same energy as that of the reactants.
C. The reactants of hydrolysis have less energy than that of the products.
D. None of the above
6. Choose the correct change in free energy in the following conversion. (The products have higher free energy than the reactants)
Adenosine + Phosphate —> AMP
A. —14 kJ/mol
B. —34 kJ/mol
C. +14 kJ/mol
D. —40 kJ/mol
7. Phosphoric acid is a:
A. monoprotic acid.
B. diprotic acid.
C. triprotic acid.
D. strong acid.