March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 7th Edition (2013)
Part I. Introduction
Chapter 6. Mechanisms and Methods of Determining Them
6.I. Marcus Theory
It is often useful to compare the reactivity of one compound with that of similar compounds. The real goal is to find out how a reaction coordinate (and in particular the transition state) changes when one reactant molecule is replaced by a similar molecule. Marcus theory is a method for doing this.25
In this theory, the activation energy (ΔG‡) is thought of as consisting of two parts.
1. An intrinsic free energy of activation, which would exist if the reactants and products had the same ΔG°.26
This is a kinetic part, called the intrinsic barrier ![]()
2. A thermodynamic part, which arises from the ΔG° for the reaction.
The Marcus equation says that the overall ΔG‡ for a one-step reaction is27
![]()
where the term ΔGΔ stands for
![]()
wR, a work term, is the free energy required to bring the reactants together and wP is the work required to form the successor configuration from the products.
For a reaction of the type AX + B → BX, the intrinsic barrier28 ![]() is taken to be the average ΔG‡ for the two symmetrical reactions
is taken to be the average ΔG‡ for the two symmetrical reactions
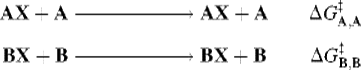
so that
![]()
One type of process that can successfully be treated by the Marcus equation is the SN2 mechanism (Sec. 10.A.i).
![]()
When R is CH3 the process is called methyl transfer.29 For such reactions, the work terms wR and wP are assumed to be very small compared to ΔG°, and can be neglected, so that the Marcus equation simplifies to
![]()
The Marcus equation allows ΔG‡ for RX + Y → RY + X to be calculated from the barriers of the two symmetrical reactions RX + X → RX + X and RY + Y → RY + Y. The results of such calculations are generally in agreement with the Hammond postulate.
Marcus theory can be applied to any single-step process where something is transferred from one particle to another. It was originally derived for electron transfers,30 and then extended to transfers of H+ (see Sec. 8.D), H−,31 and H√32, as well as methyl transfers.