March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 7th Edition (2013)
Part I. Introduction
Chapter 6. Mechanisms and Methods of Determining Them
6.J. Methods of Determining Mechanisms33
There are a number of commonly used methods for determining mechanisms.34 In most cases, one method is not sufficient, and the problem is generally approached from several directions.
6.J.i. Identification of Products
Obviously any mechanism proposed for a reaction must account for all the products obtained and for their relative proportions, including products formed by side reactions. Incorrect mechanisms for the von Richter reaction(Reaction 13-30) were accepted for many years because it was not realized that nitrogen was a major product. A proposed mechanism cannot be correct if it fails to predict the products in approximately the observed proportions. For example, any mechanism for the reaction
![]()
that fails to account for the formation of a small amount of ethane cannot be correct (see Reaction 14-1), and any mechanism proposed for the Hofmann rearrangement (Reaction 18-13):
![]()
must account for the fact that the carbonyl carbon is lost as CO2.
6.J.ii. Determination of the Presence of an Intermediate
Intermediates are postulated in many mechanisms, and the presence or absence of an intermediate is essential information. There are several methods, none of them foolproof,35 for attempting to learn whether or not an intermediate is present and, if so, its structure. All methods are experimental, and an intermediate must be detected in one way or another, often by isolation or trapping.
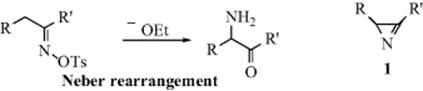
1. Isolation of an Intermediate. It is sometimes possible to isolate an intermediate from a reaction mixture by stopping the reaction after a short time or by the use of very mild conditions. For example, in the Neber rearrangement (Reaction 18-12) the intermediate 1 (an azirene)36 has been isolated. If it can be shown that the isolated compound gives the same product when subjected to the reaction conditions, and at a rate no slower than the starting compound, this constitutes strong evidence that the reaction involves that intermediate, although it is not conclusive, since the compound may arise by an alternate path and by coincidence give the same product.
2. Detection of an Intermediate. In many cases an intermediate cannot be isolated, but can be detected by normal IR, ReactIR,37 NMR, or other spectra.38 The detection by Raman spectra of NO2+ was regarded as strong evidence that this is an intermediate in the nitration of benzene (see Reaction 11-2). Free radical and triplet intermediates can often be detected by ESR and by CIDNP (see Chap 5). Free radicals (as well as radical ions and EDA complexes) can also be detected by a method that does not rely on spectra. In this method, a double-bond compound is added to the reaction mixture, and its fate traced.39 One possible result is cis–trans conversion. For example, cis-stilbene is isomerized to the trans isomer in the presence of RS√ radicals, by this mechanism:
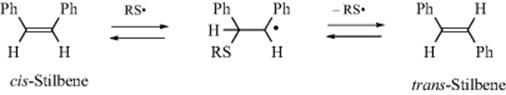
Since the trans isomer is more stable than the cis, the reaction does not go the other way, and the detection of the isomerized product is evidence for the presence of the RS√ radicals.
3. Trapping of an Intermediate. In some cases, the suspected intermediate is known to be one that reacts in a given way with a certain compound. The intermediate can then be trapped by running the reaction in the presence of that compound. For example, benzynes (Sec. 13.A.iii) react with dienes in the Diels–Alder reaction (15-60). In any reaction where a benzyne is a suspected intermediate, the addition of a diene and the detection of the Diels–Alder adduct indicate that the benzyne was probably present.
4. Addition of a Suspected Intermediate. If a certain intermediate is suspected, and if it can be obtained by other means, then under the same reaction conditions it should give the same products. This kind of experiment can provide conclusive negative evidence: if the correct products are not obtained, the suspected compound is not an intermediate. However, if the correct products are obtained, this is not conclusive since they may arise by coincidence. The von Richter reaction (Reaction 13-30) provides a good example here too. For many years, it had been assumed that an aryl cyanide was an intermediate, since cyanides are easily hydrolyzed to carboxylic acids (Reaction 16-4). Indeed, p-chlorobenzonitrile was shown to give p-chlorobenzoic acid under normal von Richter conditions.40 However, when the experiment was repeated with 1-cyanonaphthalene, no 1-naphthoic acid was obtained, although 2-nitronaphthalene gave 13% 1-naphthoic acid under the same conditions.41 This proved that 2-nitronaphthalene must have been converted to 1-naphthoic acid by a route that does not involve 1-cyanonaphthalene. It also showed that even the conclusion that p-chlorobenzonitrile was an intermediate in the conversion of m-nitrochlorobenzene to p-chlorobenzoic acid must now be suspect, since it is not likely that the mechanism would substantially change in going from the naphthalene to the benzene system.
6.J.iii. The Study of Catalysis42
Many organic reactions are slow in the absence of a catalyst. Acid-catalyzed reactions are prevalent for example. Once it is known that a reaction is subject to catalysis, much information about the mechanism of a reaction can be obtained from a knowledge of which substances catalyze the reaction, which inhibit it, and which do neither. Of course, just as a mechanism must be compatible with the products, so must it be compatible with its catalysts. In general, catalysts perform their actions by providing an alternate pathway for the reaction in which ΔG‡ is less than it would be without the catalyst. Catalysts do not change ΔG.
6.J.iv. Isotopic Labeling43
Molecules that have been isotopically labeled can be used to trace the path of the reaction, which may provide much useful mechanistic information. For example, in the reaction
![]()
does the CN group in the product come from the CN in the BrCN? The use of ![]() supplied the answer, since
supplied the answer, since ![]() gave radioactive RCN.44 This surprising result saved a lot of labor, since it ruled out a mechanism involving the replacement of CO2 by CN (see Reaction 16-94). Other radioactive isotopes are also frequently used as tracers, but even stable isotopes can be used. An example is the hydrolysis of esters
gave radioactive RCN.44 This surprising result saved a lot of labor, since it ruled out a mechanism involving the replacement of CO2 by CN (see Reaction 16-94). Other radioactive isotopes are also frequently used as tracers, but even stable isotopes can be used. An example is the hydrolysis of esters
![]()
Which bond of the ester is broken, the acyl–O or the alkyl–O bond? The answer is found by the use of ![]() . If the acyl–O bond breaks, the labeled oxygen will appear in the acid; otherwise it will be in the alcohol (see Reaction 16-59). Although neither compound is radioactive, the one that contains
. If the acyl–O bond breaks, the labeled oxygen will appear in the acid; otherwise it will be in the alcohol (see Reaction 16-59). Although neither compound is radioactive, the one that contains ![]() can be determined by submitting both to mass spectrometry. In a similar way, deuterium can be used as a label for hydrogen. In this case, mass spectrometry is not the only option since IR and
can be determined by submitting both to mass spectrometry. In a similar way, deuterium can be used as a label for hydrogen. In this case, mass spectrometry is not the only option since IR and ![]() and
and ![]() NMR45 spectra can be used to determine when deuterium has been substituted for hydrogen.
NMR45 spectra can be used to determine when deuterium has been substituted for hydrogen.
In the labeling technique, it is not generally necessary to use completely labeled compounds. Partially labeled material is usually sufficient.
6.J.v. Stereochemical Evidence46
If the products of a reaction are capable of existing in more than one stereoisomeric form, the form that is obtained may give information about the mechanism.47 For example, Walden48 discovered that (+)-malic acid gives (−)-chlorosuccinic acid when treated with PCl5 and the (+) enantiomer when treated with SOCl2, showing that the mechanisms of these apparently similar conversions could not be the same (see Sec. 10.A.i and 10.D). Much useful information has been obtained about nucleophilic substitution, elimination, rearrangement, and addition reactions from this type of experiment. The isomers involved need not be enantiomers. Thus, the fact that cis-2-butene treated with KMnO4 gives meso-2,3-butanediol and not the racemic mixture is evidence that the two OH groups attack the double bond from the same side (see Reaction 15-48).
6.J.vi. Kinetic Evidence49
The rate of a homogeneous reaction50 is the rate of disappearance of a reactant or appearance of a product. The rate nearly always changes with time, since it is usually proportional to concentration and the concentration of reactants decreases with time. However, the rate is not always proportional to the concentration of all reactants. In some cases, a change in the concentration of a reactant produces no change at all in the rate, while in other cases the rate may be proportional to the concentration of a substance (a catalyst) that does not even appear in the stoichiometric equation. A study of which reactants affect the rate often tells a good deal about the mechanism.
If the rate is proportional to the change in concentration of only one reactant (A), the rate law (the rate of change of concentration of A with time t) is
![]()
where k is the rate constant for the reaction.51 There is a minus sign because the concentration of A decreases with time. A reaction that follows such a rate law is called a first-order reaction. The units of k for a first-order reaction are reciprocal second (s−1). The rate of a second-order reaction is proportional to the concentration of two reactants, or to the square of the concentration of one:
![]()
For a second-order reaction, the units are L mol−1 s−1 or some other units expressing the reciprocal of concentration or pressure per unit time interval.
Similar expressions can be written for third-order reactions. A reaction whose rate is proportional to [A] and to [B] is said to be first order in A and in B, second order overall. A reaction rate can be measured in terms of any reactant or product, but the rates so determined are not necessarily the same. For example, if the stoichiometry of a reaction is 2A + B → C + D then, on a molar basis, A must disappear twice as fast as B, so that −d[A]/dt and −d[B]/dt are not equal but the former is twice as large as the latter.
The rate law of a reaction is an experimentally determined fact. The rate law leads to an understanding of the molecularity, which may be defined as the number of molecules that come together to form the activated complex. It is obvious that if it is known how many (and which) molecules take part in the activated complex, a good deal is known about the mechanism. The experimentally determined rate order is not necessarily the same as the molecularity. Any reaction, no matter how many steps are involved, has only one rate law, but each step of the mechanism has its own molecularity. For reactions that take place in one step (reactions without an intermediate), the order is the same as the molecularity. A first-order, one-step reaction is always unimolecular; a one-step reaction that is second order in A always involves two molecules of A; if it is first order in A and in B, then a molecule of A reacts with one of B, and so on. For reactions that take place in more than one step, the order for each step is the same as the molecularity for that step. This fact enables us to predict the rate law for any proposed mechanism, although the calculations may get lengthy at times.52 If any one step of a mechanism is considerably slower than all the others (this is usually the case), the rate of the overall reaction is essentially the same as that of the slow step, which is consequently called the rate-determining step.53
For reactions that take place in two or more steps, two broad cases can be distinguished:
1. The first step is slower than any subsequent step and is consequently rate determining. In such cases, the rate law simply includes the reactants that participate in the slow step. For example, if the reaction A + 2B → C has the mechanism
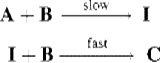
where I is an intermediate, the reaction is second order, with the rate law
![]()
2. When the first step is not rate determining, determination of the rate law is usually much more complicated. For example, consider the mechanism
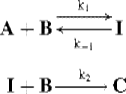
where the first step is a rapid attainment of equilibrium, followed by a slow reaction to give C. The rate of disappearance of A is
![]()
Both terms must be included because A is being formed by the reverse reaction, as well as being used up by the forward reaction. This equation is of very little help as it stands since the concentration of the intermediate cannot be measured. However, the combined rate law for the formation and disappearance of I is
![]()
This equation is of little help unless the assumption is made that the concentration of I does not change with time, since it is an intermediate that is used up (going either to A + B or to C) as fast as it is formed. This assumption, called the assumption of the steady state,54 enables d[I]/dt to be set equal to zero, and hence to solve for [I] in terms of the measurable quantities [A] and [B]:
![]()
Inserting this value for [I] into the original rate expression gives
![]()
Note that this rate law is valid whatever the values of k1, k−1, and k2. However, our original hypothesis was that the first step was faster than the second, or that
![]()
Since the first step is an equilibrium
![]()
this gives
![]()
Canceling [I] gives
![]()
Neglecting k2[B] in comparison with k−1 gives
![]()
The overall rate is thus third order: first order in A and second order in B. Incidentally, if the first step is rate determining (as was the case in the preceding paragraph), then
![]()
which is the same rate law deduced from the rule that where the first step is rate determining, the rate law includes the reactants that participate in that step.
It is possible for a reaction to involve A and B in the rate-determining step, although only [A] appears in the rate law. This occurs when a large excess of B is present, say 100 times the molar quantity of A. In this case, the complete reaction of A uses up only 1 equiv of B, leaving 99 equiv. It is not easy to measure the change in concentration of B with time in such a case, and it is seldom attempted, especially when B is also the solvent. Since [B], for practical purposes, does not change with time, the reaction appears to be first order in A although actually both A and B are involved in the rate-determining step. This is often referred to as a pseudo-first-order reaction. Pseudo-order reactions can also come about when one reactant is a catalyst whose concentration does not change with time because it is replenished as fast as it is used up and when a reaction is conducted in a medium that keeps the concentration of a reactant constant, for example, in a buffer solution where H+ or ![]() is a reactant. Pseudo-first-order conditions are frequently used in kinetic investigations for convenience in experimentation and calculations.
is a reactant. Pseudo-first-order conditions are frequently used in kinetic investigations for convenience in experimentation and calculations.
What is actually being measured is the change in concentration of a product or a reactant with time. Many methods have been used to make such measurements.55 The choice of a method depends on its convenience and its applicability to the reaction being studied. Among the most common methods are the following:
1. Periodic or Continuous Spectral Readings. In many cases, the reaction can be carried out in the cell while it is in the instrument. Then all that is necessary is that the instrument be read, periodically or continuously. Among the methods used are IR and UV spectroscopy, polarimetry, NMR, and ESR.56
2. Quenching and Analyzing. A series of reactions can be set up and each stopped in some way (perhaps by suddenly lowering the temperature or adding an inhibitor) after a different amount of time has elapsed. The materials are then analyzed by spectral readings, titrations, chromatography, polarimetry, or any other method.
3. Removal of Aliquots at Intervals. Each aliquot is then analyzed as in method 2.
4. Measurement of Changes in Total Pressure, for Gas-Phase Reactions.57
5. Calorimetric Methods. The output or absorption of heat can be measured at time intervals.
Special methods exist for kinetic measurements of very fast reactions.58
A graph is usually obtained that shows the change in concentration with time. Interpretation59 is required to obtain a rate law and a value of k. If a reaction obeys simple first- or second-order kinetics, the interpretation is generally not difficult. For example, for a concentration at the start = A0, the first-order rate law
![]()
can be integrated between the limits t = 0 and t = t to give
![]()
Therefore, if a plot of ln [A] against t is linear, the reaction is first order and k can be obtained from the slope. For first-order reactions, it is customary to express the rate not only by the rate constant k, but also by the half-life, which is the time required for one-half of any given quantity of a reactant to be used up. Since the half-life ![]() is the time required for [A] to reach Ao/2,:
is the time required for [A] to reach Ao/2,:
![]()
so that
![]()
For the general case of a reaction first order in A and first order in B, second order overall, integration is complicated, but it can be simplified if equimolar amounts of A and B are used, so that Ao = Bo. In this case,
![]()
is equivalent to
![]()
Integrating as before gives
![]()
Thus, under equimolar conditions, if a plot of 1/[A] against t is linear, the reaction is second order with a slope of k. It is obvious that the same will hold true for a reaction second order in A.60
Although many reaction-rate studies do give linear plots, which are easily interpreted, the results in many other studies are not so simple. In some cases, a reaction may be first order at low concentrations but second order at higher concentrations. In other cases, fractional orders are obtained, and even negative orders. The interpretation of complex kinetics often requires much skill and effort. Even where the kinetic data are relatively simple, there is often a problem in interpreting the data because of the difficulty of obtaining sufficiently precise measurements.61
Nuclear magnetic resonance spectra can be used to obtain kinetic information in a completely different manner from that mentioned above. This method, which involves the study of NMR line shapes,62 depends on the fact that NMR spectra have an inherent time factor: If a proton changes its environment less rapidly than ~ 103 times s−1, an NMR spectrum shows a separate peak for each position the proton assumes. For example, if the rate of rotation around the C–N bond of N,N-dimethylacetamide is slower than 103 rotations per second, the two N-methyl groups appear as a separate signal with different chemical shifts indicating that they are not equivalent, one being cis to the oxygen and the other trans to the acyl methyl group. However, if the environmental change takes place more rapidly than ~ 103 times per second, only one signal is found, at a chemical shift that is the weighted average of the two individual positions. In many cases, two or more signals are found at low temperatures, but as the temperature is increased, the lines coalesce because the interconversion rate increases with temperature and passes the 103 per second mark. From studies of the way line shapes change with temperature it is often possible to calculate rates of reactions and of conformational changes. This method is not limited to changes in proton line shapes, but can also be used for other atoms that give NMR and ESR spectra (Sec. 5.C.i).
Several types of mechanistic information can be obtained from kinetic studies.
1. Information can be obtained from the order of a reaction. Which molecules and how many take part in the rate-determining step may be determined. Such knowledge is very useful and often essential in elucidating a mechanism. For any mechanism that can be proposed for a given reaction, a corresponding rate law can be calculated by the methods discussed in the beginning of this section. If the experimentally obtained rate law fails to agree with this, the proposed mechanism is wrong. However, it is often difficult to relate the order of a reaction to the mechanism, especially when the order is fractional or negative. It is frequently the case that two or more proposed mechanisms for a reaction are kinetically indistinguishable, that is, they predict the same rate law.
2. Probably the most useful data obtained kinetically are the rate constants themselves. They are important since they can relate the effect on the rate of a reaction of changes in the structure of the reactants (see Chapter 9), the solvent,63 the ionic strength, the addition of catalysts, and so on.
3. If the rate is measured at several temperatures, in most cases a plot of ln k against l/T (T stands for absolute temperature) is nearly linear64 with a negative slope, and fits the equation
![]()
where R is the gas constant and A is a constant called the frequency factor. This permits the calculation of Ea, which is the Arrhenius activation energy of the reaction. Now ΔH‡ can then be obtained by
![]()
It is also possible to use these data to calculate ΔS‡ by the formula65
![]()
for energies in calorie units. For joule units, the formula is
![]()
One then obtains ΔG‡ from ΔG‡ = ΔH‡ − TΔS‡.
6.J.vii. Isotope Effects
When a hydrogen atom in a reactant molecule is replaced by deuterium, there is often a change in the rate. Such changes are known as deuterium isotope effects66 and are expressed by the ratio kH/kD. The ground-state vibrational energy (called the zero-point vibrational energy) of a bond depends on the mass of the atoms and is lower when the reduced mass is higher.67 Therefore, D–C, D–O, D–N bonds, and so on, have lower energies in the ground state than the corresponding H–C, H–O, H–N bonds, and so on. Complete dissociation of a deuterium bond consequently requires more energy than that for a corresponding hydrogen bond in the same environment (Fig. 6.4). If a H–C, H–O, or H–N bond is not broken at all in a reaction or is broken in a non-rate-determining step, substitution of deuterium for hydrogen causes no change in the rate (see below for an exception to this statement), but if the bond is broken in the rate-determining step, the rate must be lowered by the substitution.
![]()
Fig. 6.4 A C–D bond has a lower zero point than a corresponding C–H bond; thus the dissociation energy is higher.
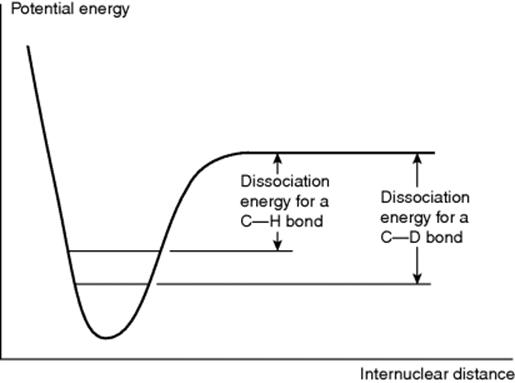
This provides a valuable diagnostic tool for determination of mechanism. For example, in the bromination of acetone (Reaction 12-4) the fact that the rate is independent of the bromine concentration led to the postulate that the rate-determining step was prior to tautomerization of the acetone:
![]()
In turn, the rate-determining step of the tautomerization involves cleavage of a C–H bond (see Reaction 12-3). Thus there should be a substantial isotope effect if deuterated acetone is brominated. In fact, kH/kD was found to be ~ 7.68 Deuterium isotope effects usually range from 1 (no isotope effect at all) to ~ 7 or 8, although in a few cases, larger69 or smaller values have been reported.70 Values of kH/kD < 1 are called inverse isotope effects. Isotope effects are greatest when, in the transition state, the hydrogen is symmetrically bonded to the atoms between which it is being transferred.71 Also, calculations show that isotope effects are at a maximum when the hydrogen atom in the transition state is on the straight line connecting the two atoms between which it is being transferred, and that for sufficiently nonlinear configurations, they decrease to kH/kD = 1–2.72 Of course, in open systems there is no reason for the transition state to be nonlinear, but this is not the case in many intramolecular mechanisms, (e.g., in a 1,2-migration of a hydrogen)
To measure isotope effects, it is not always necessary to prepare deuterium-enriched starting compounds. It can also be done by measuring the change in deuterium concentration at specific sites between a compound containing deuterium in natural abundance and the reaction product, using a high field NMR instrument.73
The substitution of tritium for hydrogen gives isotope effects that are numerically larger. Isotope effects have also been observed with other elements, but they are much smaller, ~ 1.02–1.10. For example, ![]() for the reaction of methoxide with benzyl bromide is 1.053.74 Although they are small, heavy-atom isotope effects can be measured quite accurately and are often very useful.75
for the reaction of methoxide with benzyl bromide is 1.053.74 Although they are small, heavy-atom isotope effects can be measured quite accurately and are often very useful.75
![]()
Deuterium isotope effects have been found even where it is certain that the C–H bond does not break at all in the reaction. Such effects are called secondary isotope effects,76 the term primary isotope effect being reserved for the type discussed previously. Secondary isotope effects can be divided into α and β effects. In a β secondary isotope effect, substitution of deuterium for hydrogen β to the position of bond breaking slows the reaction. An example is solvolysis of 2-bromopropane to 2-propanol,where kH/kD was found to be 1.34.77 The cause of β isotope effects has been a matter of much controversy, but they are most likely due to hyperconjugation effects in the transition state. The effects are greatest when the transition state has considerable carbocation character.78 Although the C–H bond in question is not broken in the transition state, the carbocation is stabilized by hyperconjugation (Sec. 2.M) involving this bond. Because of hyperconjugation, the difference in vibrational energy between the C–H bond and the C–D bond in the transition state is less than it is in the ground state, so the reaction is slowed by substitution of deuterium for hydrogen.
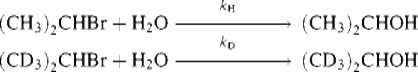
Support for hyperconjugation as the major cause of β isotope effects is the fact that the effect is greatest when D is anti to the leaving group79 (because of the requirement that all atoms in a resonance system be coplanar, planarity of the D–C–C–X system would most greatly increase the hyperconjugation), and the fact that secondary isotope effects can be transmitted through unsaturated systems.80 There is evidence that at least some β isotope effects are steric in origin81 (e.g., a CD3 group has a smaller steric requirement than a CH3 group) and a field-effect explanation has also been suggested (CD3 is apparently a better electron donor than CH382), but hyperconjugation is the most probable cause in most instances.83 Part of the difficulty in attempting to explain these effects is their small size, ranging only as high as ~ 1.5.84 Another complicating factor is that they can change with temperature. In one case,85kH/kD was 1.00 ± 0.01 at 0 °C, 0.90 ± 0.01 at 25 °C, and 1.15 ± 0.09 at 65 °C. Whatever the cause, there seems to be a good correlation between β secondary isotope effects and carbocation character in the transition state. They are thus a useful tool for probing mechanisms.
The other type of secondary isotope effect results from a replacement of hydrogen by deuterium at the carbon containing the leaving group. These so-called secondary isotope effects are varied, with values so far reported86ranging from 0.87 to 1.26.87 These effects are also correlated with carbocation character. Nucleophilic substitutions that do not proceed through carbocation intermediates (SN2 reactions) have an isotope effect near unity.88 Those that do involve carbocations (SN1 reactions) have higher isotope effects, which depend on the nature of the leaving group.89 The accepted explanation for a isotope effects is that one of the bending C–H vibrations is affected by the substitution of D for H more or less strongly in the transition state than in the ground state.90 Depending on the nature of the transition state, this may increase or decrease the rate of the reaction. The α isotope effects on SN2 reactions can vary with concentration,91 an effect attributed to a change from a free nucleophile to one that is part of an ion pair92 (see Sec. 10.G.ii). This illustrates the use of secondary isotope effects as a means of studying transition state structure. The γ secondary isotope effects have also been reported.93
Another kind of isotope effect is the solvent isotope effect.94 Reaction rates often change when the solvent is changed from H2O to D2O or from ROH to ROD. These changes may be due to any of three factors or a combination of all of them.
1. The solvent may be a reactant. If an O–H bond of the solvent is broken in the rate-determining step, there will be a primary isotope effect. If the molecules involved are D2O or D3O+ there may also be a secondary effect caused by the O–D bonds that are not breaking.
2. The substrate molecules may become labeled with deuterium by rapid hydrogen exchange, and then the newly labeled molecule may become cleaved in the rate-determining step.
3. The extent or nature of solvent–solute interactions may be different in the deuterated and non-deuterated solvents; this may change the energies of the transition state, and hence the activation energy of the reaction. These are secondary isotope effects. Two physical models for this third factor have been constructed.95
It is obvious that in many cases the first and third factors at least, and often the second, are working simultaneously. Attempts have been made to separate them.96
The methods described in this chapter are not the only means of determining mechanisms. A detailed examination of the literature, coupled with well-planned experiments is the best way to devise an approach to the mechanism of a given reaction.
Notes
1. Perspectives on Structure and Mechanism in Organic Chemistry, Carroll, F.A., Wiley, 2010; Arrow-Pushing in Organic Chemistry: An Easy Approach to Understanding Reaction Mechanisms, Levy, D.E., Wiley–Interscience, 2008; Guidebook to Mechanism in Organic Chemistry, 6th Edition, Sykes, P., Prentice Hall, 1996.
2. For a classification of pericyclic reactions, see Hendrickson, J.B. Angew. Chem. Int. Ed. 1974, 13, 47. Also see, Fleming, I. Pericyclic Reactions, Oxford University Press, Oxford, 1999.
3. For a discussion of the activation strain model of chemical reactivity, see van Zeist, W.-J.; Bickelhaupt, F.M. Org. Biomol. Chem., 2010, 8, 3118.
4. For calculations of long-chain alkane energies see Song, J.-W.; Tsuneda, T.; Sato, T.; Hirao, K. Org. Lett. 2010, 12, 1440.
5. To initiate a reaction of a mixture of H2 and O2, energy must be added such as by striking a match.
6. Strictly speaking, this is an energy profile for a reaction of the type XY + Z → X + YZ. However, it may be applied, in an approximate way, to other reactions.
7. For a review of reaction coordinates and structure–energy relationships, see Grunwald, E. Prog. Phys. Org. Chem. 1990, 17, 55.
8. For a discussion of transition states, see Laidler, K.J. J. Chem. Educ. 1988, 65, 540.
9. See Kreevoy, M.M.; Truhlar, D.G. in Bernasconi, C.F. Investigation of Rates and Mechanisms of Reactions, 4th ed. (Vol. 6 of Weissberger, A. Techniques of Chemistry), pt. 1, Wiley, NY, 1986, pp. 13–95; Moore, J.W.; Pearson, R.G. Kinetics and Mechanism, 3rd ed., Wiley, NY, 1981, pp. 137–181; Klumpp, G.W. Reactivity in Organic Chemistry, Wiley, NY, 1982; pp. 227–378. See Zevatskii, Y.E.; Samoilov, D.V. Russ. J. Org. Chem. 2007, 43, 483.
10. See Donahue, N.M. Chem. Rev. 2003, 103, 4593.
11. For a monograph on diffusion-controlled reactions, see Rice, S.A. Comprehensive Chemical Kinetics, Vol. 25 (edited by Bamford, C.H.; Tipper, C.F.H.; Compton, R.G.); Elsevier: NY, 1985.
12. As will be seen in Chapter 17, elimination is also possible with some molecules if the hydrogen is oriented syn, instead of anti, to the chlorine atom. Of course, this orientation also requires a considerable loss of entropy.
13. See De Tar, D.F.; Luthra, N.P.J. Am. Chem. Soc. 1980, 102, 4505; Mandolini, L. Bull. Soc. Chim. Fr. 1988, 173. For a related discussion, see Menger, F.M. Acc. Chem. Res. 1985, 18, 128.
14. See Nakagaki. R.; Sakuragi, H.; Mutai, K. J. Phys. Org. Chem. 1989, 2, 187; Mandolini, L. Adv. Phys. Org. Chem. 1986, 22, 1; Winnik, M.A. Chem. Rev. 1981, 81, 491; Valters, R. Russ. Chem. Rev. 1982, 51, 788.
15. The values for ring sizes 4, 5, and 6 are from Mandolini, L. J. Am. Chem. Soc. 1978, 100, 550; the others are from Galli, C.; Illuminati, G.; Mandolini, L.; Tamborra, P. J. Am. Chem. Soc. 1977, 99, 2591. See also, Illuminati, G.; Mandolini, L. Acc. Chem. Res. 1981, 14, 95. See, however, Benedetti, F.; Stirling, C.J.M. J. Chem. Soc. Perkin Trans. 2 1986, 605.
16. See laser femtochemistry: Zewall, A.H.; Bernstein, R.B. Chem. Eng. News 1988, 66, No. 45 (Nov. 7), 24–43. For another method, see Collings, B.A.; Polanyi, J.C.; Smith, M.A.; Stolow, A.; Tarr, A.W. Phys. Rev. Lett. 1987, 59, 2551.
17. See Smith, M.B. Organic Synthesis, 3rd ed., Wavefunction Inc./Elsevier, Irvine, CA/London, England, 2010, pp. 564–572.
18. Baldwin, J.E. J. Chem. Soc. Chem. Commun. 1976, 734; Baldwin, J.E. in Further Perspectives in Organic Chemistry (Ciba Foundation Symposium 53), Elsevier, Amsterdam, The Netherlands, 1979, pp. 85–99. See also, Baldwin, J.E.; Thomas, R.C.; Kruse, L.I.; Silberman, L. J. Org. Chem. 1977, 42, 3846; Baldwin, J.E.; Lusch, M.J. Tetrahedron 1982, 38, 2939; Fountain, K.R.; Gerhardt, G. Tetrahedron Lett. 1978, 3985.
19. For some exceptions to the rule in this case, see Trost, B.M.; Bonk, P.J. J. Am. Chem. Soc. 1985, 107, 1778; Torres, L.E.; Larson, G.L. Tetrahedron Lett. 1986, 27, 2223.
20. Johnson, C.D. Accts. Chem. Res. 1997, 26, 476.
21. Baldwin, J.E.; Kruse, L.I. J. Chem. Soc. Chem. Commun. 1977, 233.
22. Baldwin, J.E.; Lusch, M.J. Tetrahedron 1982, 38, 2939.
23. See Klumpp, G.W. Reactivity in Organic Chemistry, Wiley, NY, 1982, pp. 36–89.
24. Hammond, G.S. J. Am. Chem. Soc. 1955, 77, 334. For a discussion, see Farcasiu, D. J. Chem. Educ. 1975, 52, 76.
25. See Albery, W.J. Annu. Rev. Phys. Chem. 1980, 31, 227; Kreevoy, M.M.; Truhlar, D.G. in Bernasconi, C.F. Investigation of Rates and Mechanisms of Reactions, 4th ed. (Vol. 6 of Weissberger, A. Techniques of Chemistry), pt. 1, Wiley, NY, 1986, pp. 13–95.
26. The parameter ΔG° is the standard free energy; that is, ΔG at atmospheric pressure.
27. Albery, W.J.; Kreevoy, M.M. Adv. Phys. Org. Chem. 1978, 16, 87, pp. 98–99.
28. See Lee, I. J. Chem. Soc. Perkin Trans. 2 1989, 943, Chem. Soc. Rev. 1990, 19, 133.
29. See Albery, W.J.; Kreevoy, M.M. Adv. Phys. Org. Chem. 1978, 16, 87. See also, Lee, I. J. Chem. Soc., Perkin Trans. 2 1989, 943; Lewis, E.S.; McLaughlin, M.L.; Douglas, T.A. J. Am. Chem. Soc. 1985, 107, 6668; Lewis, E.S. Bull. Soc. Chim. Fr. 1988, 259.
30. Marcus, R.A. J. Phys. Chem. 1963, 67, 853, Annu. Rev. Phys. Chem. 1964, 15, 155; Eberson, L. Electron Transfer Reactions in Organic Chemistry; Springer: NY, 1987.
31. Kim, D.; Lee, I.H.; Kreevoy, M.M. J. Am. Chem. Soc. 1990, 112, 1889 and references cited therein.
32. See, for example, Dneprovskii, A.S.; Eliseenkov, E.V. J. Org. Chem. USSR 1988, 24, 243.
33. The Investigation of Organic Reactions and their Mechanisms Maskill, H. (Ed.), Blackwell, Oxford, 2006.
34. See Bernasconi, C.F. Investigation of Rates and Mechanisms of Reactions, 4th ed. (Vol. 6 of Weissberger, A. Techniques of Chemistry), 2 pts., Wiley: NY, 1986; Carpenter, B.K. Determination of Organic Reaction Mechanisms, Wiley: NY, 1984.
35. For a discussion, see Martin, R.B. J. Chem. Educ. 1985, 62, 789.
36. See Gentilucci, L.; Grijzen, Y.; Thijs, L.; Zwanenburg, B. Tetrahedron Lett. 1995, 36, 4665.
37. ReactIR uses mid-range IR spectroscopy for the identification and monitoring of critical reaction species and follows the changes in the reaction on a second-by-second basis. For applications, see Stead, D.; Carbone, G.; O'Brien, P.; Campos, K.R.; Coldham, I; Sanderson, A. J. Am. Chem. Soc. 2010, 132, 7260; Pippel, D.J.; Weisenburger, G.A.; Faibish, N.C.; Beak, P. J. Am. Chem. Soc. 2001, 123, 4919.; Rutherford, J.L.; Hoffmann, D.; Collum, D.B. J. Am. Chem. Soc. 2002, 124, 264.
38. See Parker, V.D. Adv. Phys. Org. Chem. 1983, 19, 131; Sheridan, R.S. Org. Photochem. 1987, 8, 159.
39. For a review, see Todres, Z.V. Tetrahedron 1987, 43, 3839.
40. Bunnett, J.F.; Rauhut, M.M.; Knutson, D.; Bussell, G.E. J. Am. Chem. Soc. 1954, 76, 5755.
41. Bunnett, J.F.; Rauhut, M.M. J. Org. Chem. 1956, 21, 944.
42. See Jencks, W.P. Catalysis in Chemistry and Enzymology, McGraw-Hill, NY, 1969; Bender, M.L. Mechanisms of Homogeneous Catalysis from Protons to Proteins, Wiley, NY, 1971; Coenen, J.W.E. Recl. Trav. Chim. Pays-Bas, 1983, 102, 57; and in Bernasconi, C.F. Investigation of Rates and Mechanisms of Reactions, 4th ed. (Vol.6 of Weissberger, A. Techniques of Chemistry), pt. 1, Wiley, NY, 1986, the articles by Keeffe, J.R.; Kresge, A.J. pp. 747–790; Haller, G.L.; Delgass, W.N. pp. 951–979.
43. See Wentrup, C. in Bernasconi, C.F. Investigation of Rates and Mechanisms of Reactions, 4th ed. (Vol. 6 of Weissberger. A. Techniques of Chemistry), pt. 1, Wiley, NY, 1986, pp. 613–661; Collins, C.J. Adv. Phys. Org. Chem.1964, 2, 3. See also, the series Isotopes in Organic Chemistry.
44. Douglas, D.E.; Burditt, A.M. Can. J. Chem. 1958, 36, 1256.
45. For a review, see Hinton, J.; Oka, M.; Fry, A. Isot. Org. Chem. 1977, 3, 41.
46. See Billups, W.E.; Houk, K.N.; Stevens, R.V. in Bernasconi, C.F. Investigation of Rates and Mechanisms of Reactions, 4th ed. (Vol. 6 of Weissberger, A. Techniques of Chemistry), pt. 1, Wiley, NY, 1986, pp. 663–746; Eliel, E.L. Stereochemistry of Carbon Compounds, McGraw-Hill, NY, 1962; Newman, M.S. Steric Effects in Organic Chemistry, Wiley, NY, 1956.
47. Bonnet, L.; Larrégaray, P.; Duguay, B.; Rayez, J.-C.; Che, D.C.; Kasai, T. Bull. Chem. Soc. Jpn. 2007, 80, 707.
48. Walden, P. Ber. 1896, 29, 136; 1897, 30, 3149; 1899, 32, 1833.
49. See Connors, K.A. Chemical Kinetics, VCH, NY, 1990; Zuman, P.; Patel, R.C. Techniques in Organic Reaction Kinetics, Wiley, NY, 1984; Drenth, W.; Kwart, H. Kinetics Applied to Organic Reactions, Marcel Dekker, NY, 1980; Hammett, L.P. Physical Organic Chemistry, 2nd ed., McGraw-Hill, NY, 1970, pp. 53–100; Gardiner Jr., W.C. Rates and Mechanisms of Chemical Reactions, W.A. Benjamin: NY, 1969; Leffler, J.E.; Grunwald, E. Rates and Equilibria of Organic Reactions, Wiley, NY, 1963; Jencks, W.P. Catalysis in Chemistry and Enzymology, McGraw-Hill, NY, 1969, pp. 555–614.
50. A homogeneous reaction occurs in one phase. Heterogeneous kinetics have been studied much less.
51. Colins, C.C.; Cronin, M.F.; Moynihan, H.A.; McCarthy, D.G. J. Chem. Soc. Perkin Trans. 1 1997, 1267.
52. For a discussion of how order is related to molecularity in many complex situations, see Szabó, Z.G. in Bamford, C.H.; Tipper, C.F.H. Comprehensive Chemical Kinetics, Vol.2, Elsevier, NY, 1969, pp. 1–80.
53. Many chemists prefer to use the term rate-limiting step or rate-controlling step for the slow step, rather than rate-determining step. See the definitions in Gold, V.; Loening, K.L.; McNaught, A.D.; Sehmi, P. IUPAC Compendium of Chemical Terminology, Blackwell Scientific Publications, Oxford, 1987, p. 337. For a discussion of rate-determining steps, see Laidler, K.J. J. Chem. Educ. 1988, 65, 250.
54. For a discussion, see Raines, R.T.; Hansen, D.E. J. Chem. Educ. 1988, 65, 757.
55. See Zuman, P.; Patel, R.C. Techniques in Organic Reaction Kinetics, Wiley, NY, 1984. See Batt, L. in Bamford, C.H.; Tipper, C.F.H. Comprehensive Chemical Kinetics, Vol. 1, Elsevier, NY, 1969, pp. 1–111.
56. For a review of ESR to measure kinetics, see Norman, R.O.C. Chem. Soc. Rev. 1979, 8, 1.
57. See le Noble, W.J. Prog. Phys. Org. Chem. 1967, 5, 207; Matsumoto, K.; Sera, A.; Uchida, T. Synthesis 1985, 1; Matsumoto, K.; Sera, A. Synthesis 1985, 999.
58. See Connors, K.A. Chemical Kinetics, VCH, NY, 1990, pp. 133–186; Zuman, P.; Patel, R.C. Techniques in Organic Reaction Kinetics, Wiley, NY, 1984, pp. 247–327; Krüger, H. Chem. Soc. Rev. 1982, 11, 227; Bernasconi, C.F. Investigation of Rates and Mechanisms of Reactions, 4th ed. (Vol. 6 of Weissberger, A. Techniques of Chemistry), pt. 2, Wiley, NY, 1986. See also, Bamford, C.H.; Tipper, C.F.H. Comprehensive Chemical Kinetics, Vol.24, Elsevier, NY, 1983.
59. See Connors, K.A. Chemical Kinetics, VCH, NY, 1990, pp. 17–131; Ritchie, C.D. Physical Organic Chemistry, 2nd ed., Marcel Dekker, NY, 1990, pp. 1–35; Zuman, P.; Patel, R.C. Techniques in Organic Reaction Kinetics,Wiley, NY, 1984; Margerison, D. in Bamford, C.H.; Tipper, C.F.H. Comprehensive Chemical Kinetics, Vol. 1, Elsevier, NY, 1969, pp. 343–421; Moore, J.W.; Pearson, R.G. Kinetics and Mechanism, 3rd ed., Wiley, NY, 1981, pp. 12–82; in Bernasconi, C.F. Investigation of Rates and Mechanisms of Reactions, 4th ed. (Vol. 6 of Weissberger, A. Techniques of Chemistry), pt. 1, Wiley, NY, 1986, the articles by Bunnett, J.F. pp. 251–372, Noyes Pub., pp. 373–423, Bernasconi, C.F. pp. 425–485, Wiberg, K.B. pp. 981–1019.
60. See Margerison, D. in Bamford, C.H.; Tipper, C.F.H. Comprehensive Chemical Kinetics, Vol. 1, Elsevier, NY, 1969, p. 361.
61. See Hammett, L.P. Physical Organic Chemistry, 2nd ed., McGraw-Hill, NY, 1970, pp. 62–70.
62. See Öki, M. Applications of Dynamic NMR Spectroscopy to Organic Chemistry, VCH, NY, 1985; Fraenkel, G. in Bernasconi, C.F. Investigation of Rates and Mechanisms of Reactions, 4th ed. (Vol. 6 of Weissberger, A. Techniques of Chemistry), pt. 2, Wiley, NY, 1986, pp. 547–604; Roberts, J.D. Pure Appl. Chem.1979, 51, 1037; Binsch, G. Top. Stereochem.1968, 3, 97.
63. For a discussion of organic reaction rate acceleration by immediate solvent evaporation, see Orita, A.; Uehara, G.; Miwa, K.; Otera, J. Chem. Commun. 2006, 4729.
64. See Blandamer, M.J.; Burgess, J.; Robertson, R.E.; Scott, J.M.W. Chem. Rev. 1982, 82, 259.
65. See Bunnett, J.F. in Bernasconi, C.F. Investigation of Rates and Mechanisms of Reactions, 4th ed. (Vol. 6 of Weissberger, A. Techniques of Chemistry), pt. 1, Wiley, NY, 1986, p. 287.
66. See Melander, L.; Saunders, Jr., W.H. Reaction Rates of Isotopic Molecules, Wiley, NY, 1980. For reviews, see Isaacs, N.S. Physical Organic Chemistry, Longman Scientific and Technical, Essex, 1987, pp. 255–281; Lewis, E.S. Top. Curr. Chem. 1978, 74, 31; Saunders, Jr., W.H. in Bernasconi, C.F. Investigation of Rates and Mechanisms of Reactions, 4th ed. (Vol. 6 of Weissberger, A. Techniques of Chemistry), pt. 1, Wiley, NY, 1986, pp. 565–611; Bell, R.P. Chem. Soc. Rev. 1974, 3, 513; Bigeleisen, J.; Lee, M.W.; Mandel, F. Annu. Rev. Phys. Chem. 1973, 24, 407; Wolfsberg, M. Annu. Rev. Phys. Chem. 1969, 20, 449. Also see Kwart, H. Acc. Chem. Res. 1982, 15, 401; Isaacs, E.S. Isot. Org. Chem. 1984, 6, 67; Thibblin, A.; Ahlberg, P. Chem. Soc. Rev. 1989, 18, 209. See also, the series Isotopes in Organic Chemistry.
67. The reduced mass μ of two atoms connected by a covalent bond is μ = m1m2/(m1 + m2).
68. Reitz, O.; Kopp, J. Z. Phys. Chem. Abt. A 1939, 184, 429.
69. For an example of a reaction with a deuterium isotope effect of 24.2, see Lewis, E.S.; Funderburk, L.H. J. Am. Chem. Soc. 1967, 89, 2322. The high isotope effect in this case has been ascribed to tunneling of the proton: See Lewis, E.S.; Robinson, J.K. J. Am. Chem. Soc. 1968, 90, 4337; Kresge, A.J.; Powell, M.F. J. Am. Chem. Soc. 1981, 103, 201; Caldin, E.F.; Mateo, S.; Warrick, P. J. Am. Chem. Soc. 1981, 103, 202. For arguments that high isotope effects can be caused by factors other than tunneling, see Thibblin, A. J. Phys. Org. Chem. 1988, 1, 161; Kresge, A.J.; Powell, M.F. J. Phys. Org. Chem. 1990, 3, 55.
70. See Sims, L.B.; Lewis, D.E. Isot. Org. Chem. 1984, 6, 161.
71. Bethell, D.; Hare, G.J.; Kearney, P.A. J. Chem. Soc. Perkin Trans. 2 1981, 684, and references cited therein. See, however, Motell, E.L.; Boone, A.W.; Fink, W.H. Tetrahedron 1978, 34, 1619.
72. More O'Ferrall, R.A. J. Chem. Soc. B 1970, 785, and references cited therein.
73. Pascal, R.A.; Baum, M.W.; Wagner, C.K.; Rodgers, L.R.; Huang, D. J. Am. Chem. Soc. 1986, 108, 6477.
74. Stothers, J.B.; Bourns, A.N. Can. J. Chem. 1962, 40, 2007. See also, Ando, T.; Yamataka, H.; Tamura, S.; Hanafusa, T. J. Am. Chem. Soc. 1982, 104, 5493.
75. For a review of carbon isotope effects, see Willi, A.V. Isot. Org. Chem. 1977, 3, 237.
76. See Westaway, K.C. Isot. Org. Chem. 1987, 7, 275; Sunko, D.E.; Hehre, W.J. Prog. Phys. Org. Chem. 1983, 14, 205; Halevi, E.A. Prog. Phys. Org. Chem. 1963, 1, 109. See McLennan, D.J. Isot. Org. Chem. 1987, 7, 393. See also, Sims, L.B.; Lewis, D.E. Isot. Org. Chem. 1984, 6, 161.
77. Leffek, K.T.; Llewellyn, J.A.; Robertson, R.E. Can. J. Chem. 1960, 38, 2171.
78. Bender, M.L.; Feng, M.S. J. Am. Chem. Soc. 1960, 82, 6318; Jones, J.M.; Bender, M.L. J. Am. Chem. Soc. 1960, 82, 6322.
79. DeFrees, D.J.; Hehre, W.J.; Sunko, D.E. J. Am. Chem. Soc. 1979, 101, 2323. See also, Siehl, H.; Walter, H. J. Chem. Soc. Chem. Commun. 1985, 76.
80. Shiner, Jr., V.J.; Kriz, Jr., G.S. J. Am. Chem. Soc. 1964, 86, 2643.
81. Carter, R.E.; Dahlgren, L. Acta Chem. Scand. 1970, 24, 633; Leffek, K.T.; Matheson, A.F. Can. J. Chem. 1971, 49, 439; Sherrod, S.A.; Boekelheide, V. J. Am. Chem. Soc. 1972, 94, 5513.
82. Halevi, E.A.; Nussim, M.; Ron, M. J. Chem. Soc. 1963, 866; Halevi, E.A.; Nussim, M. J. Chem. Soc. 1963, 876.
83. Sunko, D.E.; Szele, I.; Hehre, W.J. J. Am. Chem. Soc. 1977, 99, 5000; Kluger, R.; Brandl, M. J. Org. Chem. 1986, 51, 3964.
84. Halevi, E.A.; Margolin, Z. Proc. Chem. Soc. 1964, 174. A value for ![]() of 2.13 was reported for one case: Liu, K.; Wu, Y.W. Tetrahedron Lett. 1986, 27, 3623.
of 2.13 was reported for one case: Liu, K.; Wu, Y.W. Tetrahedron Lett. 1986, 27, 3623.
85. Halevi, E.A.; Margolin, Z. Proc. Chem. Soc. 1964, 174.
86. See Caldwell, R.A.; Misawa, H.; Healy, E.F.; Dewar, M.J.S. J. Am. Chem. Soc. 1987, 109, 6869.
87. See Harris, J.M.; Hall, R.E.; Schleyer, P.v.R. J. Am. Chem. Soc. 1971, 93, 2551.
88. For reported exceptions, see Tanaka, N.; Kaji, A.; Hayami, J. Chem. Lett. 1972, 1223; Westaway, K.C. Tetrahedron Lett. 1975, 4229.
89. Shiner, Jr., V.J.; Neumann, A.; Fisher, R.D. J. Am. Chem. Soc. 1982, 104, 354 and references cited therein.
90. Streitwieser, Jr., A.; Jagow, R.H.; Fahey, R.C.; Suzuki, S. J. Am. Chem. Soc. 1958, 80, 2326.
91. Westaway, K.C.; Waszczylo, Z.; Smith, P.J.; Rangappa, K.S. Tetrahedron Lett. 1985, 26, 25.
92. Westaway, K.C.; Lai, Z. Can. J. Chem. 1988, 66, 1263.
93. Werstiuk, N.H.; Timmins, G.; Cappelli, F.P. Can. J. Chem. 1980, 58, 1738.
94. See Alvarez, F.J.; Schowen, R.L. Isot. Org. Chem. 1987, 7, 1; Kresge, A.J.; More O'Ferrall, R.A.; Powell, M.F. Isot. Org. Chem. 1987, 7, 177; Schowen, R.L. Prog. Phys. Org. Chem. 1972, 9, 275;. See Arnett, E.M.; McKelvey, D.R. in Coetzee, J.F.; Ritchie, C.D. cited above, pp. 343–398.
95. Bunton, C.A.; Shiner, Jr., V.J. J. Am. Chem. Soc. 1961, 83, 42, 3207, 3214; Swain, C.G.; Thornton, E.R. J. Am. Chem. Soc. 1961, 83, 3884, 3890. See also, Mitton, C.G.; Gresser, M.; Schowen, R.L. J. Am. Chem. Soc. 1969, 91, 2045.
96. More O'Ferrall, R.A.; Koeppl, G.W.; Kresge, A.J. J. Am. Chem. Soc. 1971, 93, 9.