Organic Chemistry: Concepts and Applications - Headley Allan D. 2020
Oxidation Reactions in Organic Chemistry
11.5 Oxidation of Alkenes with Bond Cleavage
There are two types of oxidation reactions that proceed with cleavage of the double bond; the first occurs when using KMnO4, the same oxidizing reagent used in the previous section, but if the reaction is carried out at elevated temperatures, bond cleavage occurs. The second oxidation reaction of alkenes that occurs with bond cleavage is by using the oxidizing reagent ozone (O3), followed by reduction using Zn2+/H3O+ or (CH3)2S. As a result, this type of reaction is called ozonolysis. Reaction (11-60) gives a general representation of oxidation reactions that occur with bond cleavage.
(11-60)
As we have seen from previous reactions, the products that are actually obtained depend to a large degree on the oxidizing reagent used and reaction conditions. In this section, we will examine different oxidizing agents and reaction conditions necessary to accomplish the specific type of reactions given in Reaction (11-60). Each type of reaction will be examined in the next section, along with their mechanisms.
11.5.1 Oxidation of Alkenes with KMnO4 at Elevated Temperatures
The general oxidation of alkenes with KMnO4 in the presence of heat is shown in Reaction (11-61).
(11-61)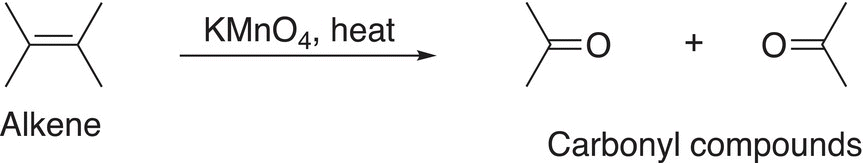
A specific example of the oxidation of an alkene using a strong oxidizing reagent at elevated temperature is shown in Reaction (11-62).
(11-62)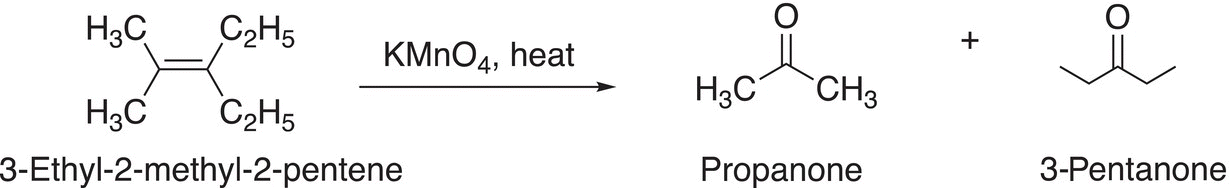
Note that if one of the carbonyl products obtained contains a hydrogen bonded to the carbonyl carbon, further oxidation occurs in the presence of the strong oxidizing reagent, KMnO4, to produce the carboxylic acid as illustrated in Reaction (11-63).
(11-63)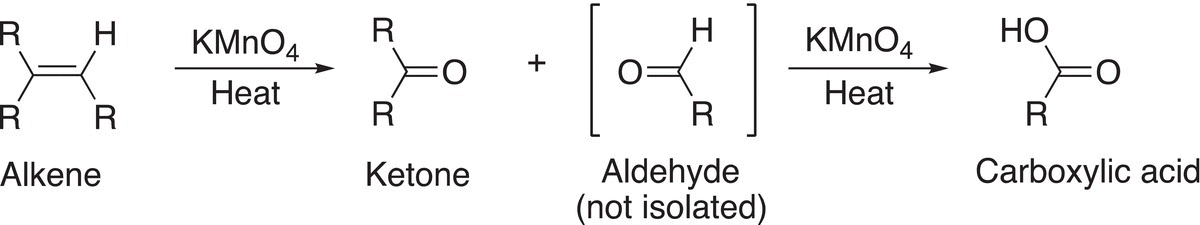
A specific example of the oxidation of an alkene that has one hydrogen bonded to the carbon—carbon double bond and a methyl group bonded to the other carbon of the double bond is shown in Reaction (11-64).
(11-64)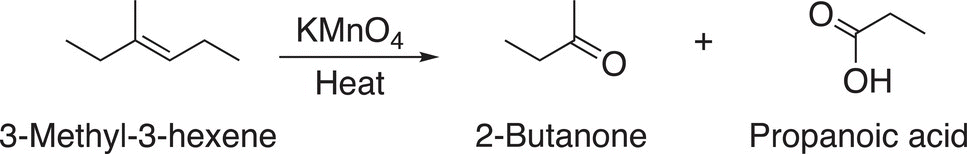
The outcome of the oxidation of this unsymmetrical alkene is that two different products are produced, one a carboxylic acid and the other a ketone. Reaction (11-65) shows the oxidation of an alkene that has one hydrogen bonded to each of the carbons of the carbon—carbon double bond. The aldehyde that is initially produced is readily oxidized into the carboxylic acid.
(11-65)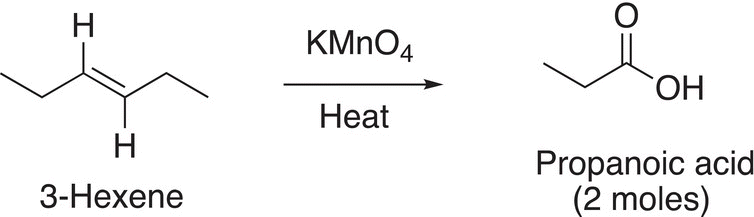
Reaction (11-66) shows the oxidation of a cyclic alkene in which the carbon—carbon double bond has a methyl and hydrogen bonded to each of the carbons. The result of this oxidation is an open chain difunctional molecule.
(11-66)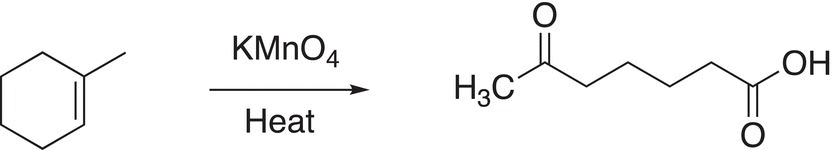
An examination of the reaction mechanism gives the rationale for the products formed. The first step of the reaction mechanism is given in Section 11.4.2, Reaction (11-54), but in the presence of heat, the intermediate decomposes as shown in Reaction (11-67).
(11-67)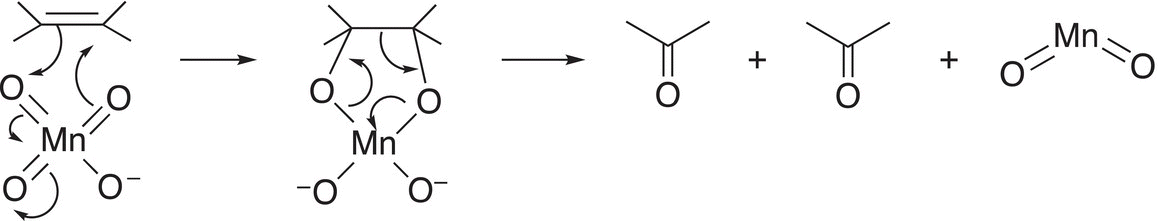
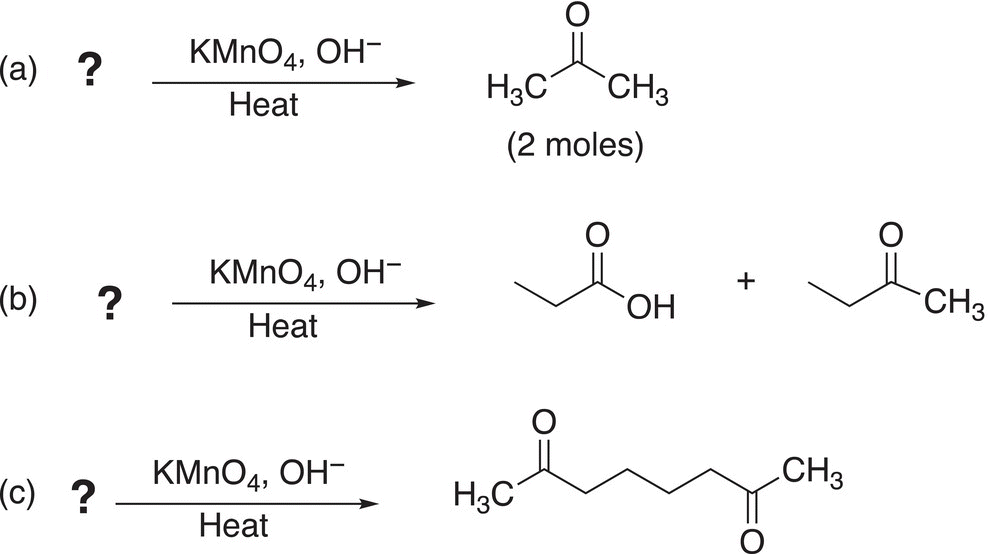
Problem 11.12
Determine the reactant alkene needed to complete the following reactions.
11.5.2 Ozonolysis of Alkenes
The oxidation of the carbon—carbon double bond with ozone (O3), followed by a reaction with dimethyl sulfide, results in the cleavage of the double bond. The reaction conditions for this oxidation reaction are mild enough that if an aldehyde is produced as a product, it is not oxidized further to form the carboxylic acidic as we have seen using KMnO4 and heat. The general reaction is shown below.
(11-68)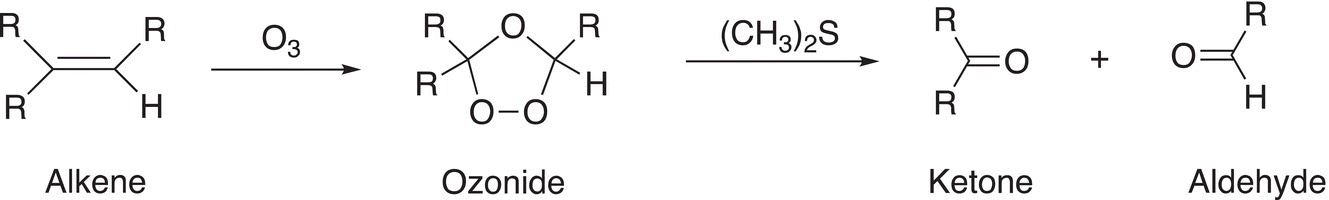
The mechanism for this reaction is shown below; this first step involves an addition of the ozone to the alkene, followed by the formation of an ozonide.
(11-69)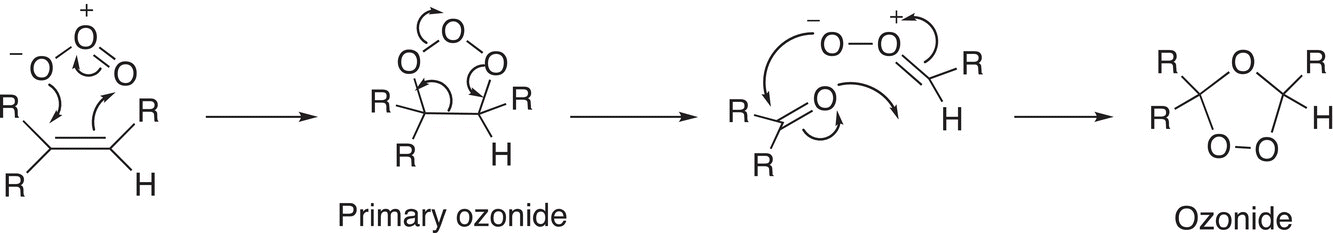
We will not go into the details of the mechanism for the reaction of the ozonide, but the general reaction is shown in Reaction (11-70).
(11-70)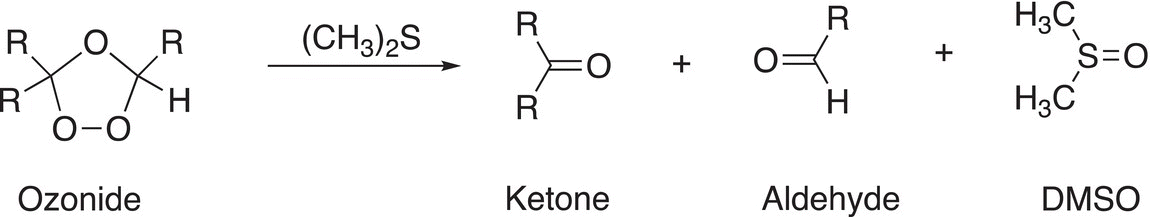
Reaction (11-71) gives an example of an ozonolysis reaction.
(11-71)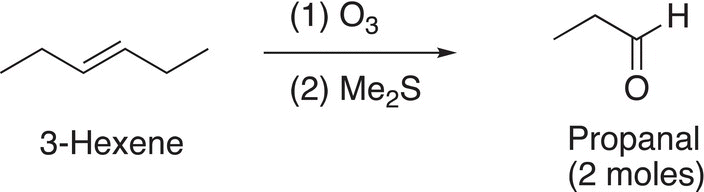
Note that there are two moles of the aldehyde produced since the starting compound is a symmetrical alkene. On the other hand, if the alkene were not symmetrical, two products result, as shown in the examples given in Reactions (11-72) and (11-73).
(11-72)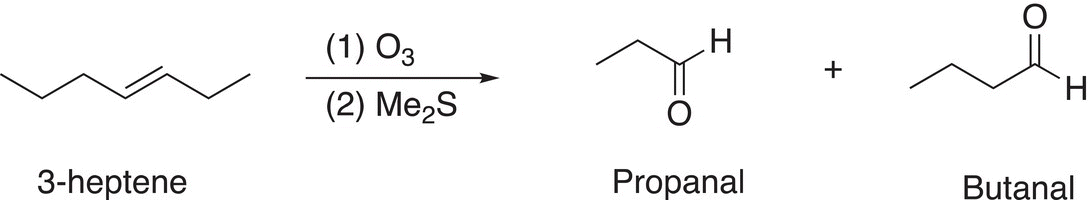
(11-73)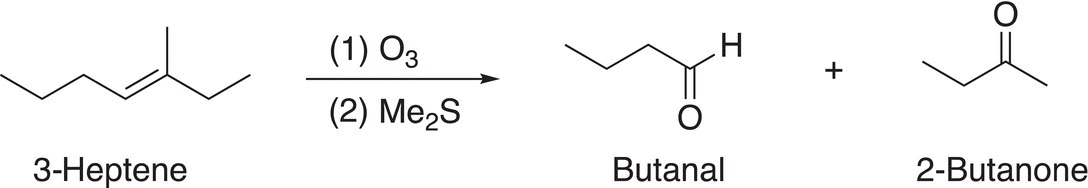
If the alkene is part of a ring, a dicarbonyl compound results, as shown in Reaction (11-74).
(11-74)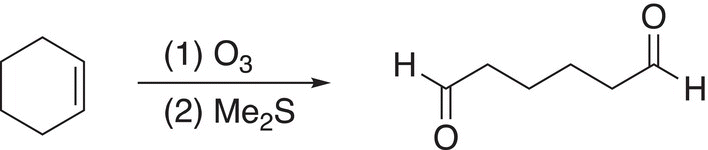
Based on the expected products of this type of reaction, you should be able to utilize this information to determine a possible starting compound. In fact, this technique is often used to determine the structure of an unknown alkene. Based on the product obtained, one can easily determine the original reactant. The next problem is designed to test your ability to make this determination.
Problem 11.13
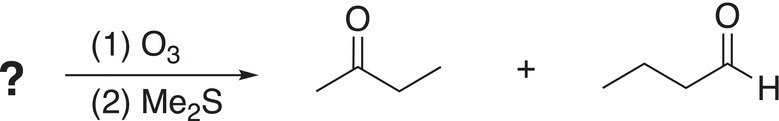
i. An unknown compound is treated by ozone, followed by Me2S using the reaction shown below, what is the structure of the reactant?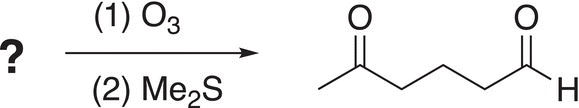
ii. An unknown compound is treated by ozone, followed by Me2S using the reaction shown below, what is the structure of the reactant?