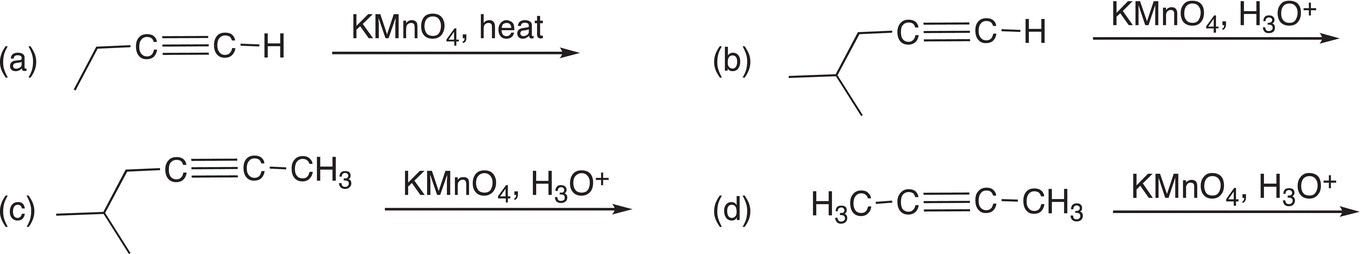Organic Chemistry: Concepts and Applications - Headley Allan D. 2020
Oxidation Reactions in Organic Chemistry
11.7 Oxidation of Alkynes
Like alkenes, alkynes can be oxidized, but as you can imagine, the oxidizing reagent has to be a very strong oxidizing agent since the carbon—carbon triple bond is a very strong covalent bond. As mentioned earlier, KMnO4 is a strong oxidizing agent, and it can be used to oxidize alkynes by adding two moles of ─OH groups across the triple bond as shown in Reaction (11-83).
(11-83)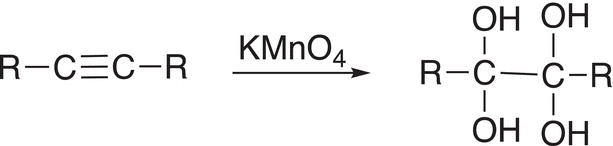
The product of Reaction (11-84) is not very stable and, in the presence of an acid, protonation of one of the OH groups occurs and converts it to a good leaving group and the result is the formation of a carbonyl functionality as shown in Reaction (11-84).
(11-84)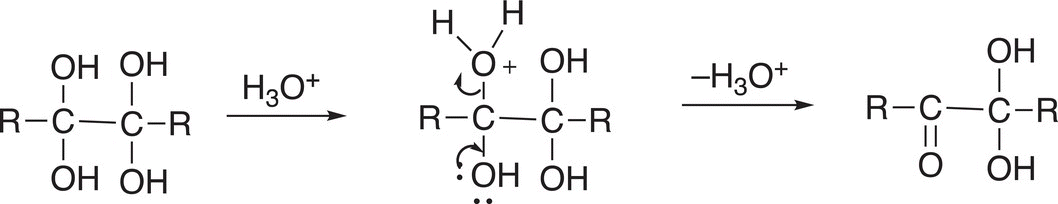
As you can imagine, a similar reaction will take place at the other carbon, which contains two OH groups, as shown in Reaction (11-85).
(11-85)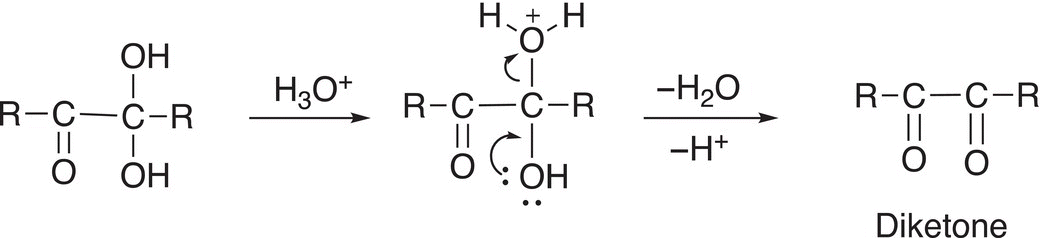
The overall reaction of an internal alkyne in the presence of an oxidizing reagent, such as KMnO4, results in a diketone as shown in the example in Reaction (11-86).
(11-86)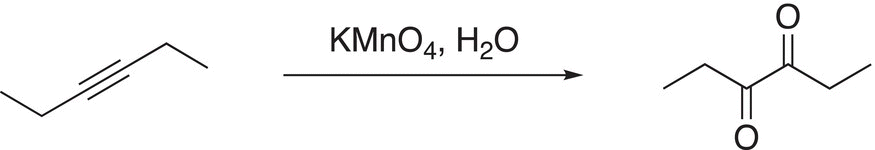
Reactions involving terminal alkynes result in α-keto acids as shown in Reactions (11-87) and (11-88).
(11-87)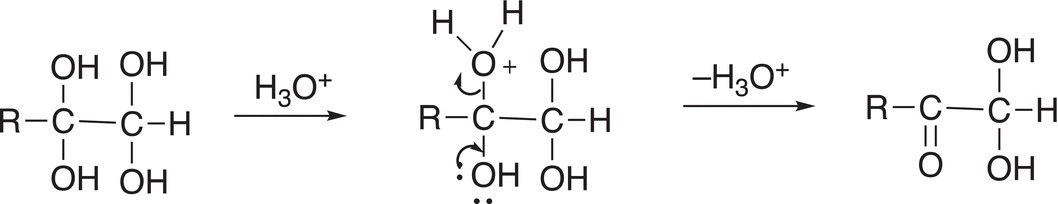
In the next step of the mechanism, one of the OH groups is protonated and converts it to a good leaving group to generate an α-keto acid as shown in Reaction (11-88).
(11-88)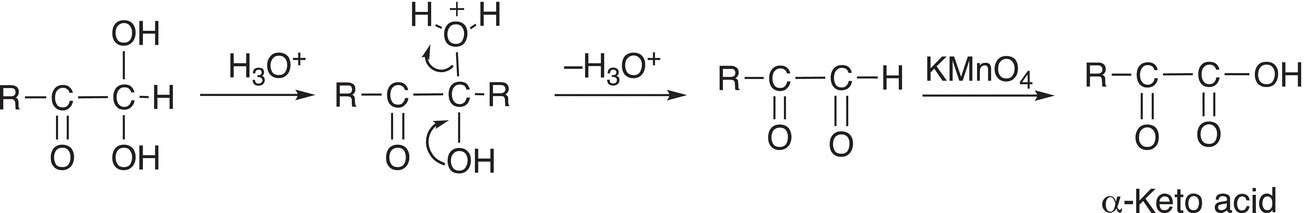
One of the most important keto acids in biology is pyruvic acid, which is important for glycolysis. Common keto acids are shown in Figure 11.1.
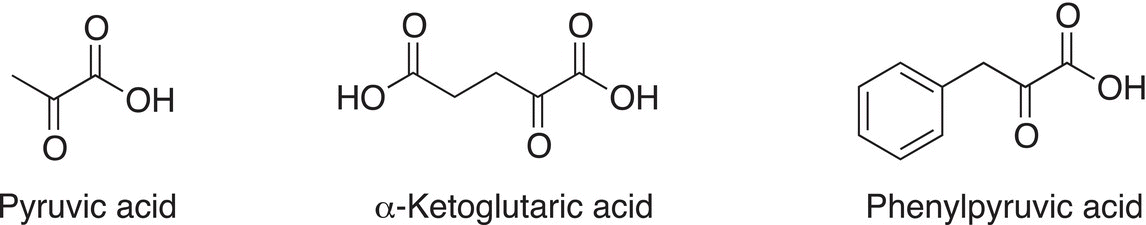
Figure 11.1 Important biological α-keto acids.
As you might expect, under more severe conditions of oxidation, alkynes can be oxidized to give the cleaved products, as shown in the reaction given in Reaction (11-89). The mechanism for such cleavage is similar to the mechanism for the cleavage of the double bonds of alkenes, which was described earlier.
(11-89)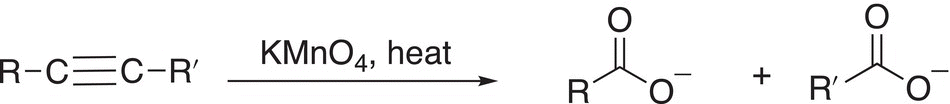
Problem 11.17
Give the products for the following reactions.