Organic Chemistry: Concepts and Applications - Headley Allan D. 2020
Free Radical Substitution Reactions Involving Alkanes
14.5 Applications of Free Radical Substitution Reactions
As pointed out in previous chapters, one of the major goals of chemists is the synthesis of specific target organic compounds by using different types of reactions that we have studied and of course making strategic selections of specific reactions. For example, we have seen that it is much better to use bromination since it is more selective, compared to chlorination if the starting compound has different types of hydrogens. On the other hand, if all the hydrogens are the same, the chlorination is a more economic choice, compared to bromination; bromine is costlier, compared to chlorine. Let us look how best to carry out the following transformation shown in Reaction (14-40) where you need to determine the appropriate reactions and reaction conditions to convert methylcyclohexane to methyl-1-cyclohexene.
(14-40)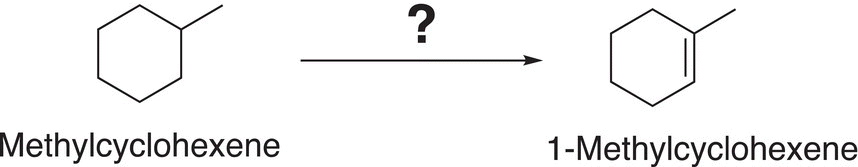
It is best to focus on the target molecule, in this case methyl-1-cyclohexene, and determine the type of reaction that can be used for its synthesis. Analysis of the target molecule reveals that the functional group contained is an alkene functionality, and we learned from Chapter 11 that elimination reactions are used to synthesize alkenes. Thus, appropriate elimination reactions for its synthesis are shown below.
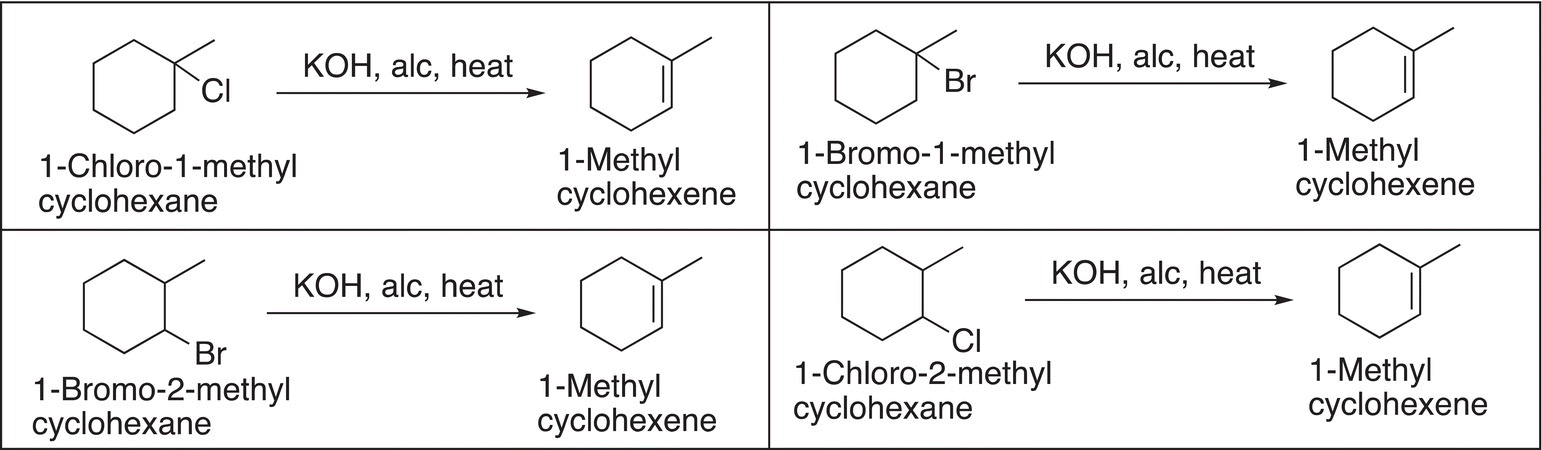
Any of these four reactions will give the desired product, but now comes the question of the appropriate selection! The challenge now comes in the synthesis of one of these alkyl halides in adequate amounts to be used in this step of the elimination reaction. The synthesis of the first reactant (1-chloro-1-methylcyclohexane) from methylcyclohexane using chlorine is not efficient, since it is not very selective and will give a low yield of this desired tertiary substitution. For the second reaction, 1-bromo-1-methylcyclohexane can be easily made via a bromination reaction to give a high yield of 1-bromo-1-methylcyclohexane since bromination is selective for a tertiary C─H substitution. For the third reaction, the substitution required to make 2-bromo-1-methylcyclohexane would be extremely difficult since the reaction would much prefer substitution at the tertiary C─H bond; the same is true for the fourth synthesis of 1-chloro-2-methylcyclohexane. Based on this information, the best elimination reaction to use for the synthesis would be the second reaction in which the highly selective bromination reaction is used for its synthesis as shown in Reaction (14-41).
(14-41)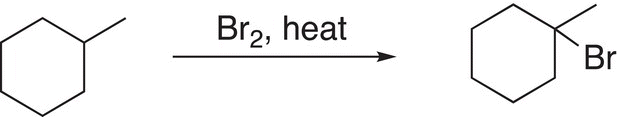
Thus, the overall sequence of reactions for the transformation given in Reaction (14-40) is shown in Reaction (14-42).
(14-42)
The same is true for the transformation shown in Reaction (14-43).
(14-43)---
The last reaction in a sequence of reactions to produce the alkene would be an elimination reaction, and the best reactant for that reaction would be 2-bromo-2-methylpropane as shown in Reaction (14-44).
(14-44)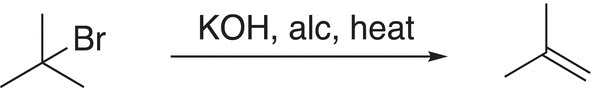
For the above target molecule, the functional group is a bromide and most specific, a tertiary bromide. We have just examined a specific type of reaction, which can perform this task of free radical halogenation of hydrocarbons. However, the appropriate hydrocarbon must be determined, in this case, one that after bromination will give the desired product is shown in Reaction (14-45).
(14-45)
For most organic reactions, only the major organic product is shown. Thus, for the above reaction, even though 1-bromo-2-methylpropane and hydrobromic acid are the other products, they are not written in the reaction. Hence, we do not have to worry about balancing the reactions.
The overall sequence of reactions would then be as shown in Reaction (14-46).
(14-46)
Problem 14.8
Starting with any saturated hydrocarbon, show how to synthesize cyclopentene.