Organic Chemistry: Concepts and Applications - Headley Allan D. 2020
Nucleophilic Substitution Reactions at sp3 Carbons
Unimolecular Substitution Reaction Mechanism (SN1 Mechanism)
An alternate mechanism for substitution reactions that was mentioned in the previous section involves the leaving group breaking away from the electrophile and taking its bonding electrons in an independent step to form a carbocation and the leaving group, as shown in Reaction (15-26).
(15-26)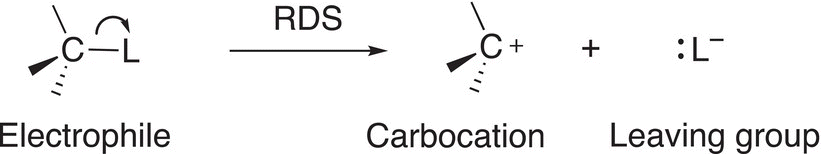
In a second step, the nucleophile attacks the carbocation formed in the initial step to produce the product as shown in Reaction (15-27).
(15-27)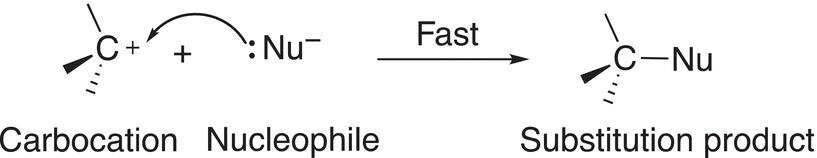
This mechanism is called an SN1 since in the first step and most important step of the reaction mechanism (also called the rate-determining step, RDS) only one molecule (the electrophile) is involved as shown in Reaction (15-26). The overall substitution reaction, which is the same using the mechanism discussed in the previous section, is shown in Reaction (15-28).
(15-28)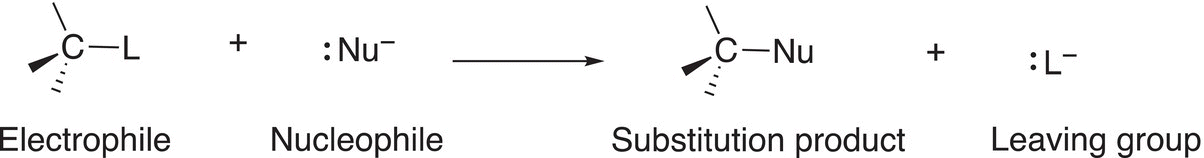
This type of reaction mechanism is described as unimolecular because as mentioned earlier, only one molecule, the electrophile, is involved in a very important step, the RDS, of the mechanism in the formation of the products. This concept of a two-step mechanism can be illustrated using an energy profile diagram as shown in Figure 15.6.
Since the RDS involves just one molecule, the mechanism for substitution reactions that proceed through this mechanism is called an SN1 — nucleophilic substitution unimolecular mechanism. The RDS is sometimes referred to as the slow step. As shown in Figure 15.6, the energy of activation for the first step of the reaction is greater than the energy of activation for the second step. Hence, the second step of the reaction is also referred to as the fast step. In the next section, we will examine substitution reactions that occur by this mechanism and the factors that influence substitution reactions that proceed via this mechanism.
15.7.1 The Nucleophile and Solvents of SN1 Reactions
In this section, we will examine the factors that influence substitution reactions that proceed via the SN1mechanism. The first step of this reaction mechanism involved the heterolytic breakage of the bond of the leaving group and the electrophile to form a carbocation and the leaving group. Since a charged carbocation is formed, dipolar protic solvents, such as water or ethanol, are typically used to promote this very important step in the reaction mechanism. Thus, polar protic solvents shown in Table 15.1 favor this reaction mechanism. In the second step of the reaction mechanism, the nucleophile attacks the carbocation to form the product. For reactions that proceed by this mechanism, typically the solvent is also the nucleophile; hence, reactions that proceed by this mechanism are also called solvolysis reactions. Since the nucleophile reacts in the second step of the mechanism, which is not the RDS, the size and nature of the nucleophile is not as important as the case for the SN2 mechanism. Thus, the rate of SN1 reactions is not dictated by the nature or concentration of the nucleophile as we saw in the SN2 reaction mechanism.
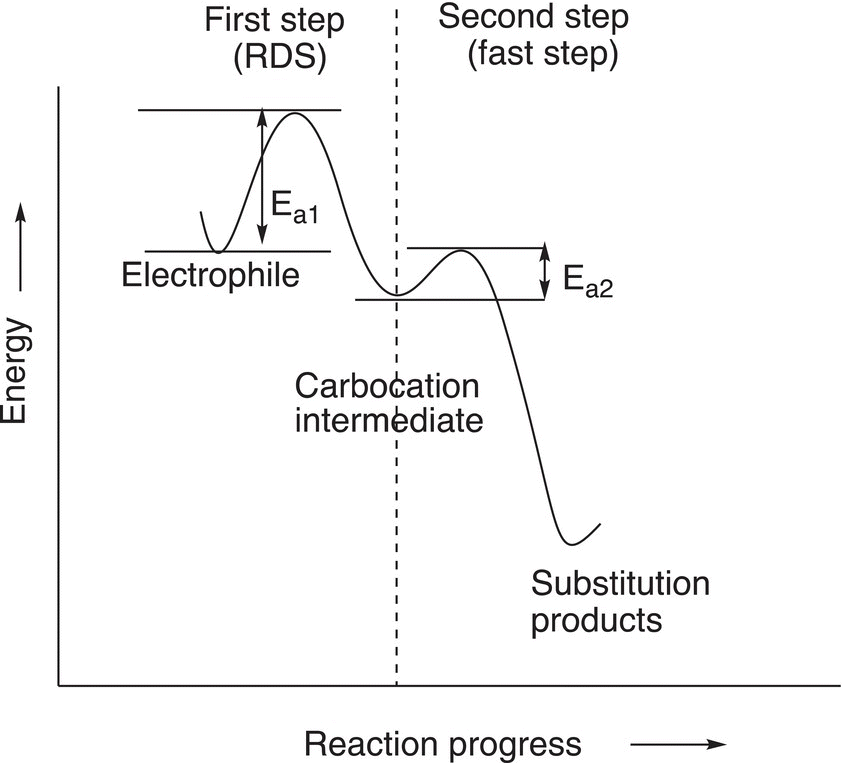
Figure 15.6 Energy profile for a typical SN1 reaction, which takes place in two steps giving a carbocation as an intermediate and then the product. Note that Ea1 for the first step (the RDS) is greater than Ea2 for the second and fast step (fast step).
Problem 15.11
i. Predict the products for the following SN1 reactions.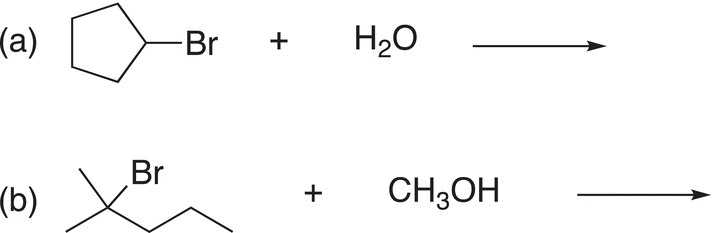
ii. Give the structure of an appropriate electrophile to complete the following SN1 reactions.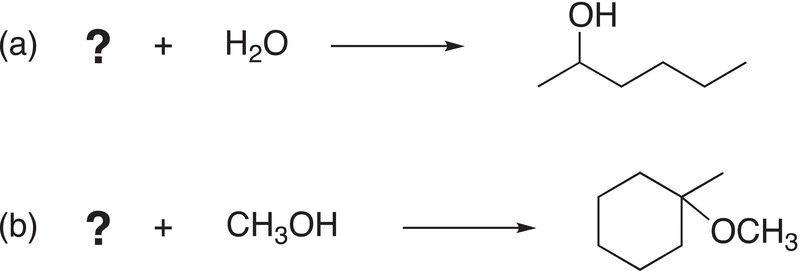
15.7.2 Stereochemistry of the Products of SN1 Reactions
For electrophiles, such as alkyl halides, that have stereogenic centers bonded to the leaving group, there is an equal mixture of both enantiomers in the product. To explain this outcome, compared to the outcome of the SN2 mechanism, we will have to look in more detail at the SN1 mechanism. The carbocation that is formed during the SN1 mechanism is sp2 hybridized and flat with a vacant p orbital perpendicular to the plane of the three bonds bonded to the carbocation. As a result, an attack of the nucleophile, in the second step of the mechanism to form a new bond with the vacant p orbital, can take place from either side of the carbocation. That is, there is an equal probability of an attack of the nucleophile at the top side or from the bottom side of the vacant p orbital of the carbocation to form the product. The result is a racemic mixture as illustrated in Figure 15.7.
As a result, it is very difficult to convert an optically active alcohol to an optically active bromide using an SN1 substitution reaction owing to the formation of a carbocation intermediate as the reaction proceeds to form products. This concept is illustrated in Reaction (15-29).
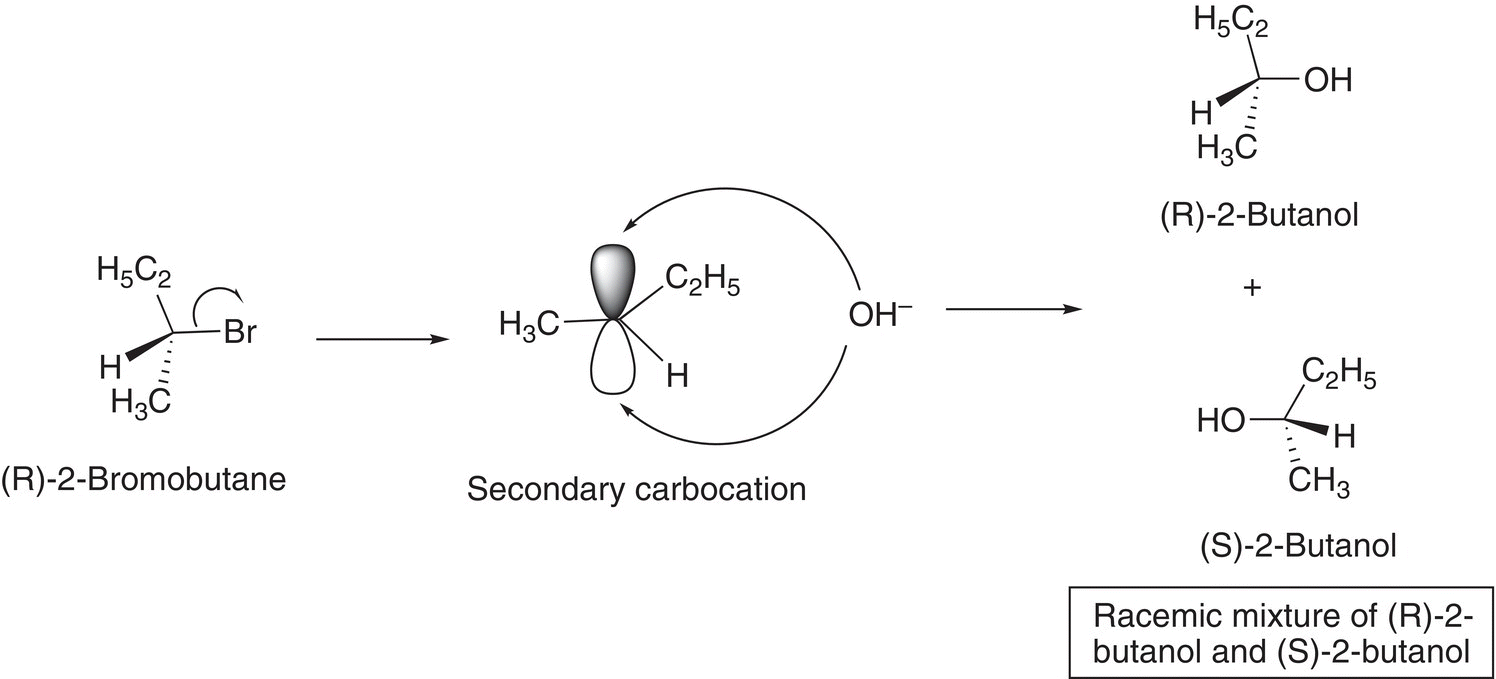
Figure 15.7 The attack of a flat carbocation by a nucleophile to give a pair of enantiomers.
(15-29)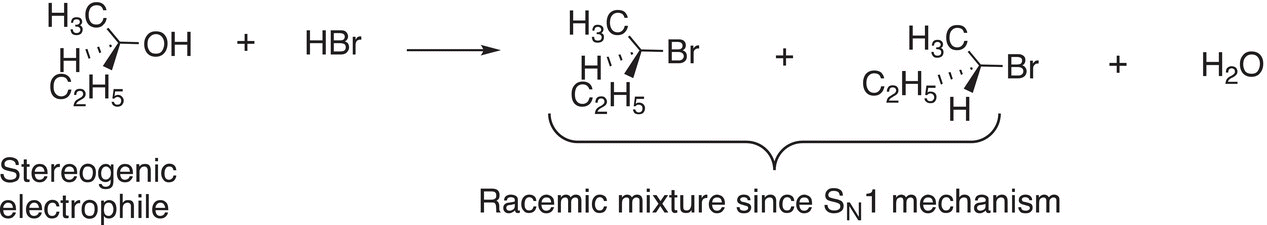
Problem 15.12
Using dashed-wedge or Fischer representation, give the structures of the racemic organic products of the SN1 reactions of CH3OH with each of the following.
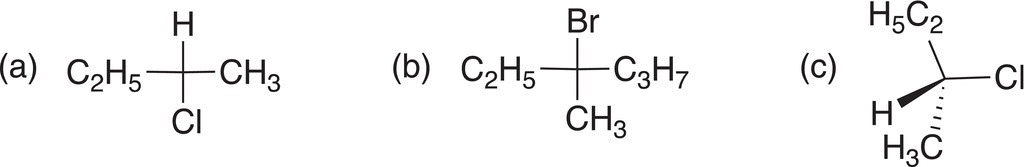
15.7.3 The Electrophile of SN1 Reactions
As mentioned earlier, the leaving groups for reactions that proceed via the SN1 mechanism are typically very good leaving groups. An exceptionally good leaving group is protonated water, which is created upon the protonation of an alcohol, to form OH2+. Protonated alcohols are produced in the presence of an acidic solution. The mechanism that is typically encountered is the SN1 mechanism if the alcohols are secondary or tertiary. The substitution reaction of alcohols and hydrogen chloride gives an alkyl chloride as the product. These reactions can be used to determine the type of an unknown alcohol. Owing to the stability of tertiary carbocations, tertiary alcohols react very fast with hydrogen chloride to produce the alkyl chloride product. Consider the substitution reaction involving a tertiary alcohol as shown in Reaction (15-30).
(15-30)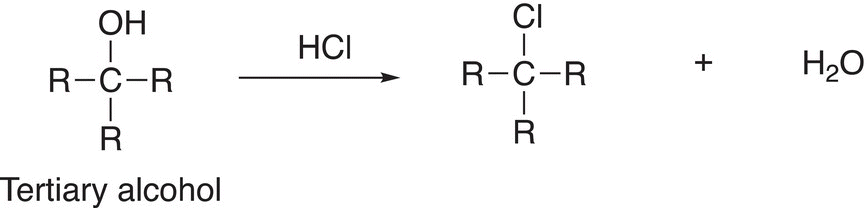
In the first step of the mechanism, the strong acid protonates the alcohol to form protonated water, which is a very good leaving group, as shown in Reaction (15-31).
(15-31)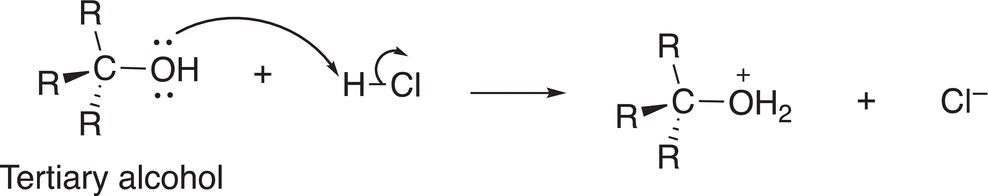
To form the carbocation ion, the extremely good H2O leaving group leaves to create the carbocation in a slow step of the mechanism as shown in Reaction (15-32).
(15-32)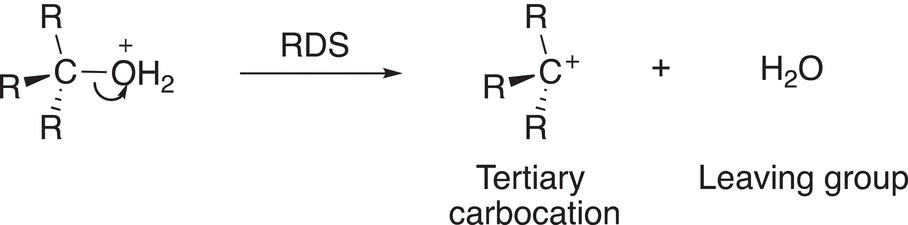
In the final step of the mechanism, the nucleophilic chloride anion reacts with the carbocation to form the final product as shown in Reaction (15-33).
(15-33)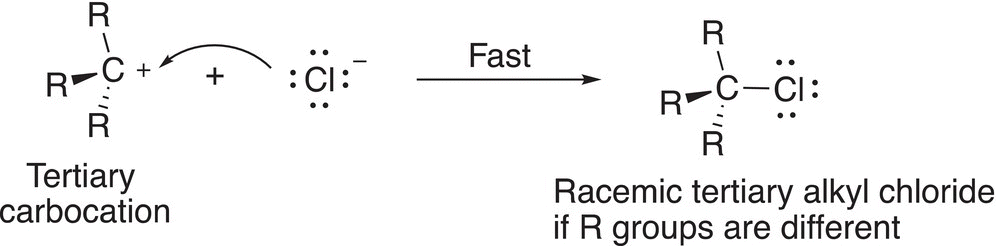
A similar analysis can be carried out for a secondary alcohol, but in these reactions, a secondary carbocation is formed, which is not as stable as a tertiary carbocation. Hence, the reaction rate is a bit slower than that of a tertiary alcohol. In the first step of the mechanism, the acid protonates the secondary alcohol to form protonated water, which leaves to create a secondary carbocation in the RDS, as shown in Reaction (15-34).
(15-34)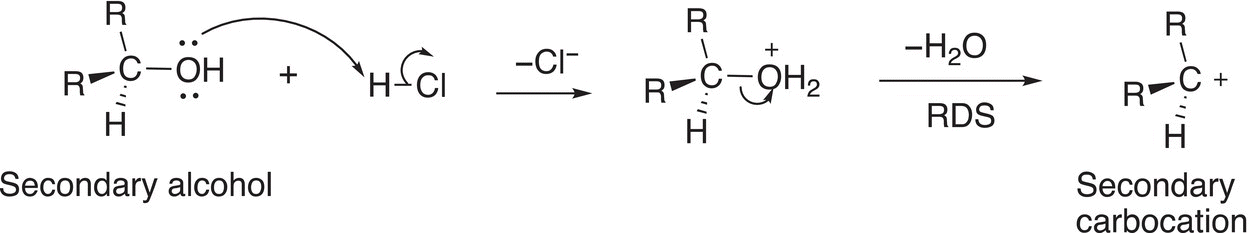
In the final step of the mechanism, the nucleophilic chloride anion reacts with the carbocation to form the final product as shown in Reaction (15-35).
(15-35)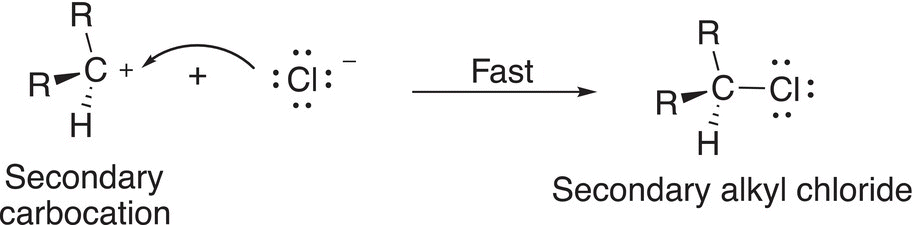
On the other hand, primary alcohols react very slowly with hydrogen chloride to form the corresponding alkyl chloride. In fact, the reaction is so slow that a catalyst, ZnCl2 is used to increase the rate of the reaction to an observable rate as shown in Reaction (15-36).
(15-36)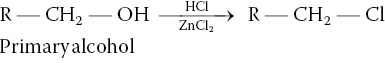
Since the rates of the reaction of different types of alcohols with hydrogen chloride are different, even in the presence of the catalyst, ZnCl2, the difference in rates can be used to determine the type of alcohol present. This test is known as the Lucas Test. The rate of the reaction of a secondary alcohol with hydrogen chloride is faster than that of primary alcohols, even in the presence of the catalyst, ZnCl2, but slower than that of tertiary alcohols.
Problem 15.13
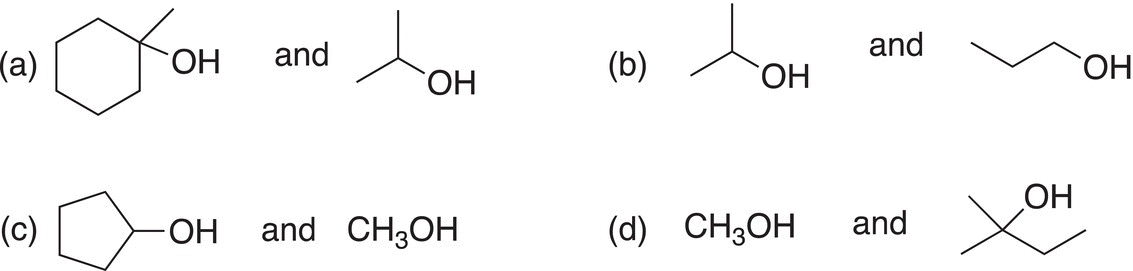
Which of the following pairs of molecules would react the fastest using a Lucas test?
Reactions that proceed via a SN1 mechanism are not the best to be used for the synthesis of a target molecule. In addition to giving racemic mixtures, rearrangement of the carbocation typically occurs giving rise to unexpected products. Reaction (15-37) gives an example of a SN1reaction that gives an unexpected product.
(15-37)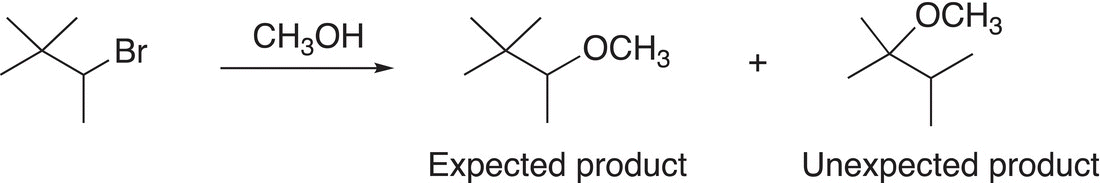
The explanation of the expected product is pretty straightforward as shown in the mechanism in Reaction (15-38).
(15-38)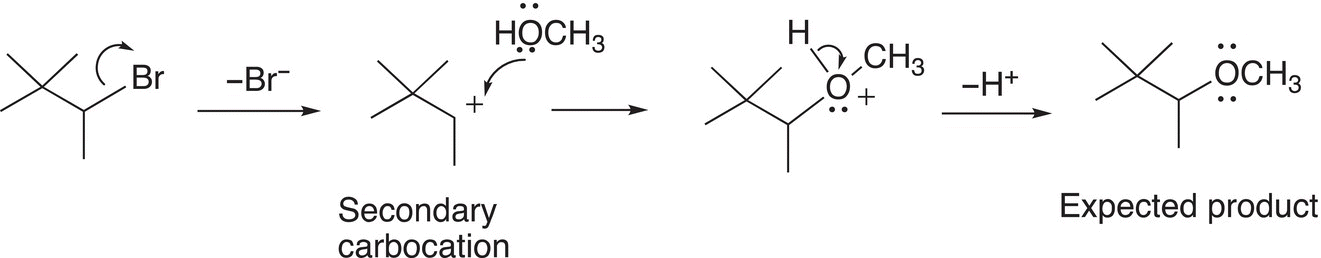
In order to fully understand and explain the appearance of the unexpected product, we will have to examine the SN1 mechanism closely and concentrate on the carbocation intermediate that is formed during the RDS. As we know, the stability of carbocations varies and depends on the number of alkyl groups that are bonded to the carbon of the carbocation. Since carbocations are positively charged species, any effect that will delocalize or stabilize this positive charge will stabilize the carbocation. Alkyl groups are considered to be electron releasing and, as a result compared to hydrogens, will stabilize carbocations. Since tertiary carbocations have three alkyl groups bonded to the carbocationic carbon, this type of carbocation is more stable than one that has one carbon and two hydrogens, i.e. secondary or primary carbocations. As we have seen from previous chapters, the relative stability of carbocations is: Tertiary (3o) > Secondary (2o) > Primary (1o) > Methyl (CH3). Carbocations often rearrange to form more stable carbocations. For the reaction given above, the initial carbocation that is formed during the course of the reaction is a secondary carbocation, which will undergo a rearrangement to form a more stable tertiary carbocation as shown in Reaction (15-39).
(15-39)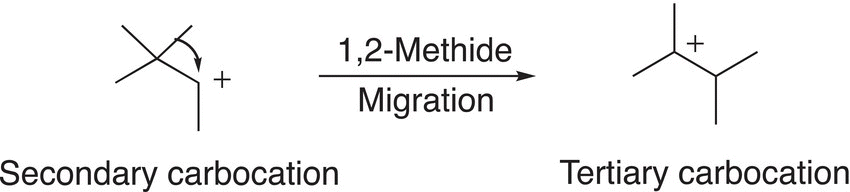
Even though the secondary carbocation is fairly stable, but the tertiary carbocation is more stable and its stability is the driving force for the rearrangement. As shown in Reaction (15-39), there is a migration of the methyl group, with its bonding electrons from the adjacent carbon, to form the tertiary carbocation. This migration is also called a methide migration and specifically 1,2 methide migration since the methide anion migrates across two carbons. The tertiary carbocation, once formed, will react with the nucleophile to form the observed major product as shown in Reaction (15-40).
(15-40)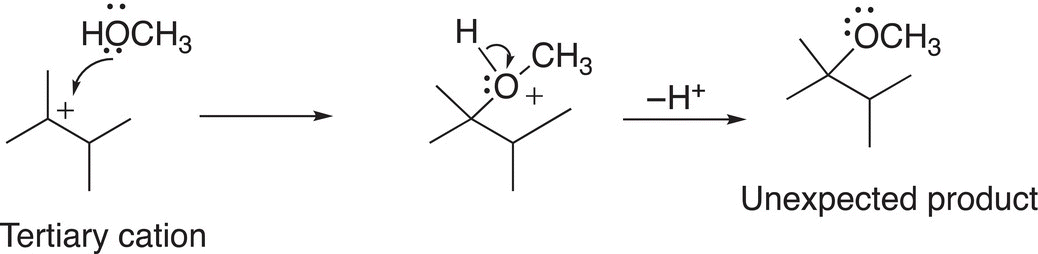
Note the hydrogen that is bonded to the oxygen nucleophilic atom becomes acidic once the nucleophile bonds to the carbocation. This hydrogen is abstracted as a proton in the final step to form the product shown. Thus, the overall reaction mechanism to form the unexpected product is shown in Reaction (15-41).
(15-41)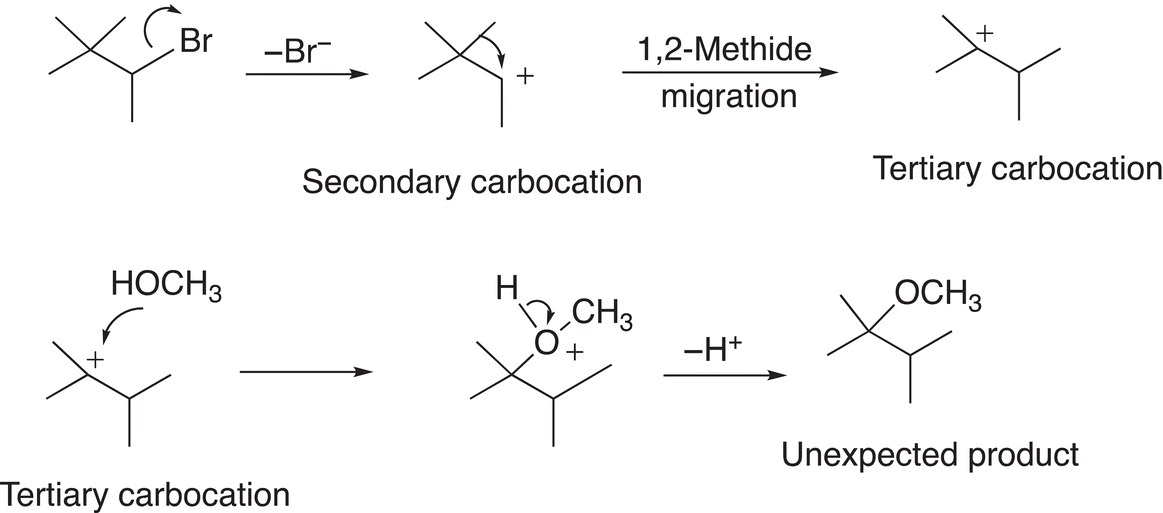
Problem 15.14
Using arrows to show electron movement, propose a mechanism to explain the products of the following reaction.
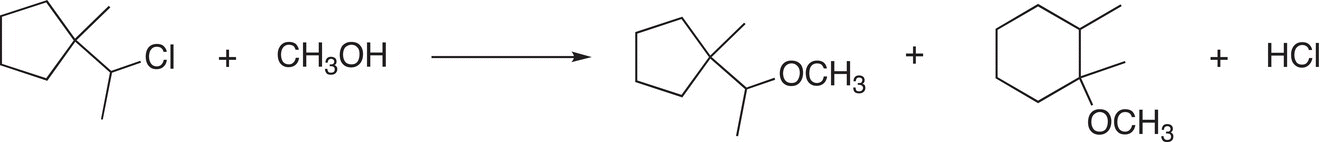
There are some electrophiles that result in very stable carbocations after the leaving group leaves. The allylic carbocation and the benzylic carbocation are examples of very stable carbocations and they are more stable than even the tertiary carbocation due to the delocalization of the electrons, as shown in Reactions (15-42) and (15-43).
(15-42)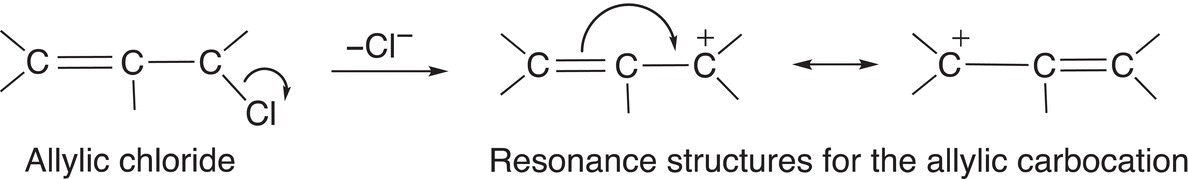
(15-43)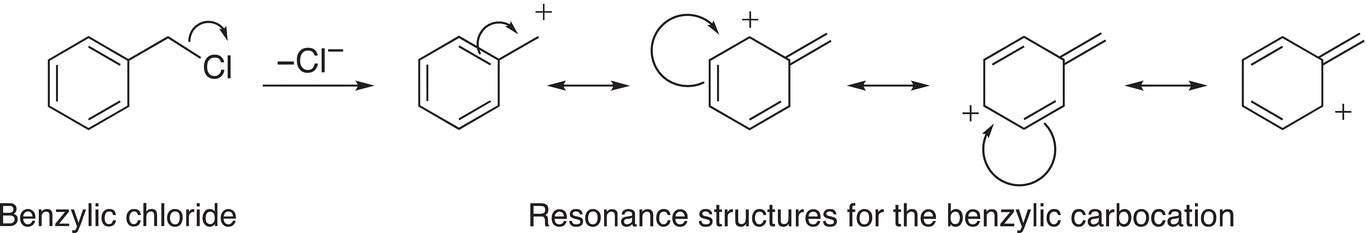
These carbocations are stable owing to the delocalization of the positive charge over the adjacent pi (π) network. Even though rearrangement does not occur with these very stable carbocations; sometimes, unexpected products are obtained as shown in Reaction (15-44).
(15-44)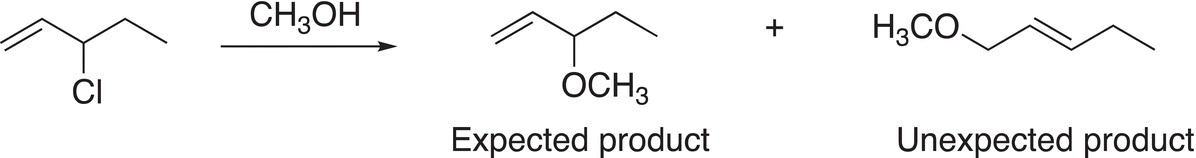
The formation of the unexpected can be explained by the reaction mechanism shown below.
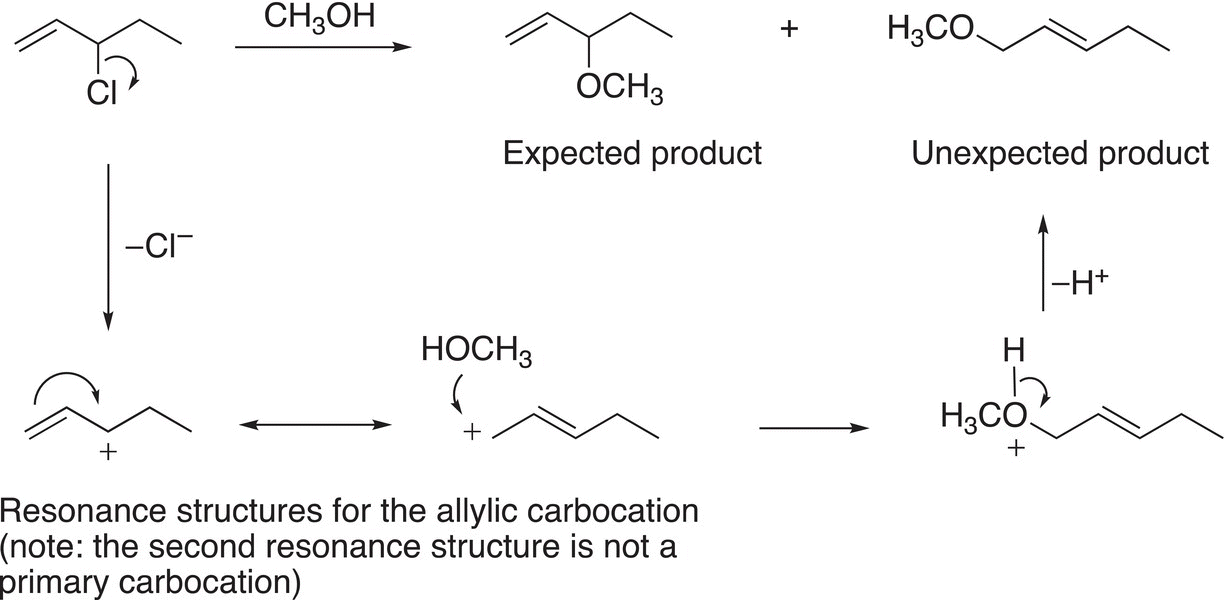
Another similar example is shown in Reaction (15-45) where there are expected and unexpected products observed.
(15-45)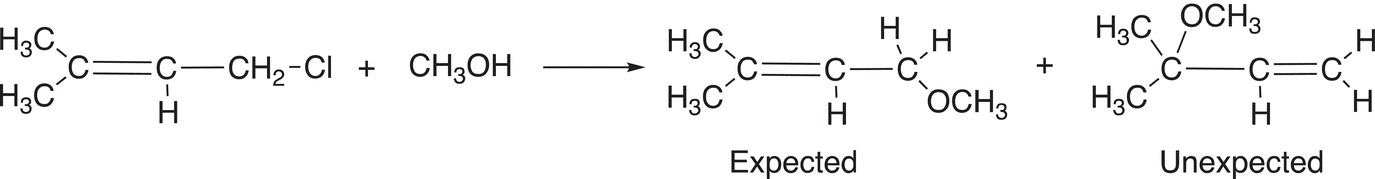
The mechanism to explain the unexpected product is shown in Reaction (15-46).
(15-46)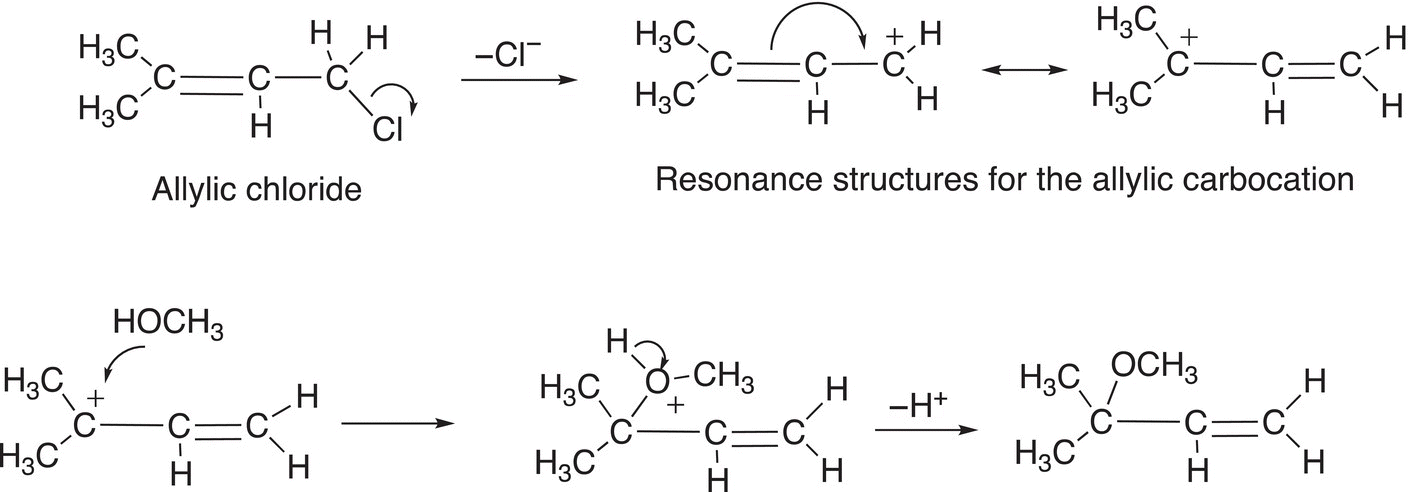
A similar unexpected product does not appear for reactions in which the benzylic carbocation is formed. The six pi (π) electrons of the ring form an exceptionally stable system that we will study in Chapter 17 and unexpected products where that cyclic six-member ring system is disturbed tend not to occur as shown in Reaction (15-47).
(15-47)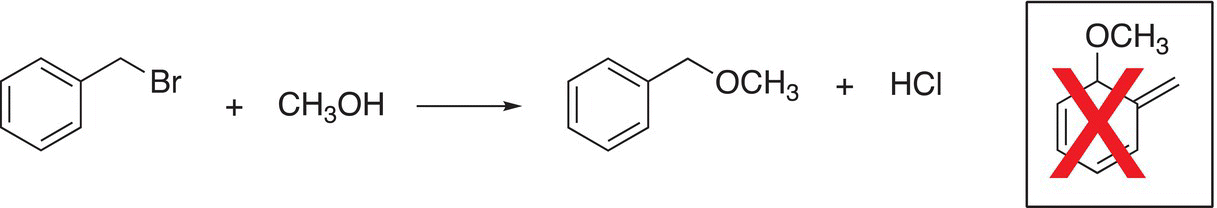
For SN1 reactions, these types of halides react very fast owing to the quick formation of the very stable intermediate carbocation that is initially formed in the RDS. These intermediate carbocations are resonance-stabilized carbocations.
Problem 15.15
Give the products (expected and unexpected) for the reaction of the alkyl halides shown below with each of the following nucleophiles: (a) H2O and (b) CH3OH.
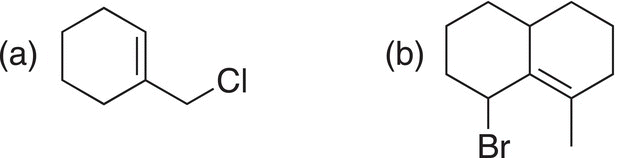
Summary: For SN1 reactions, the following factors should be considered.
1. Electrophile: The reactivity of the electrophile in SN1 reactions is: Allylic ~ benzylic > 3o > 2o > 1o > > CH3
2. Stereochemistry: An equal mixture of stereoisomers (racemic mixture) results if starting substrate is optically active
3. Nucleophile: The size of the nucleophile does not matter since attack of nucleophile occurs after the RDS and typically the solvent is the nucleophile.
4. Leaving group: For these reactions, the leaving groups are typically extremely good leaving groups, which are also weak stable conjugate bases.
5. Solvent: Solvents must be ionizing, which are typically protic solvents. Good solvents include water and alcohols, which are also typically the nucleophiles and the reactions are also called solvolysis.
6. Mechanism: Stepwise mechanism, in which the first step is the RDS and involves the formation of the carbocation, the second step is fast and involves the attack of the nucleophile on the carbocation intermediate.