Organic Chemistry: Concepts and Applications - Headley Allan D. 2020
Nucleophilic Substitution Reactions at sp3 Carbons
15.8 Applications of Nucleophilic Substitution Reactions — Synthesis
As pointed out in previous chapters, organic synthesis is a very important aspect of organic chemistry and this process typically involves forming new covalent bonds in a target product molecule. Since a single covalent bond has two electrons, there are two possibilities of making such a bond using nucleophilic substitution reactions, as illustrated in Figure 15.8.
Of course, based on your basic knowledge of chemistry, the nucleophilic reactant must be the fragment (or atom) that can best accommodate the pair of electrons and can be neutral or an anion, i.e. it must be fairly electronegative. The electrophilic fragment (or atom) is usually the partially positive carbon of a polar covalent bond to carbon, i.e. an electrophilic carbon bonded to an electronegative atom such as chlorine. In synthesis, the bonds of the functional groups of the target molecule are identified, and types of reactions are chosen that can be used to make the various functional groups of the target molecule. Thus, care must be exercised in deciding which electrophile, nucleophile, and the type of reactions to be used.
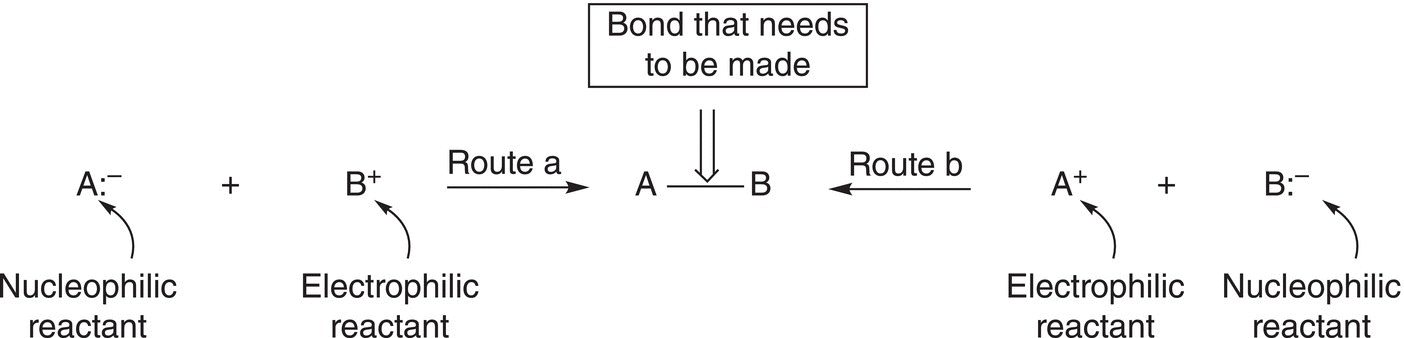
Figure 15.8 Two different ways that a single covalent bond can be synthesized.
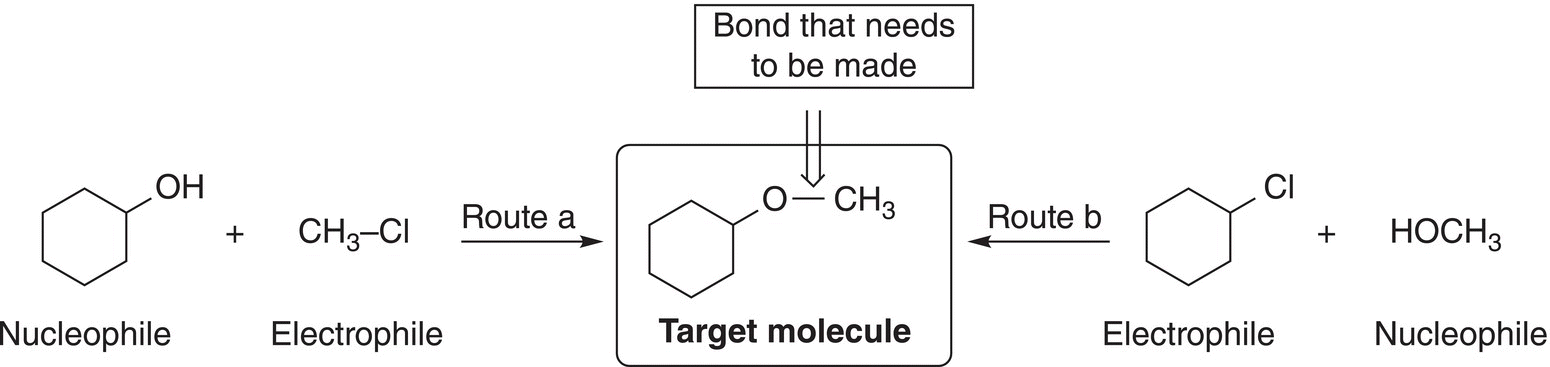
Figure 15.9 Two different ways of synthesizing the bond of the ether functionality.
15.8.1 Synthesis of Ethers
The possible ways of synthesizing an ether functionality is illustrated in Figure 15.9.
Note that you must first identify the functional group of your target molecule and then determine the bond that needs to be made that will result in that functional group. After careful analysis and application of the knowledge that we have learned thus far, route b would appears to be slightly better since it involves the formation of a secondary carbocation and route a involves the formation of a methyl carbocation which is not stable. On the other hand, the synthesis via route a could be accomplished if the conditions were right for a SN2 mechanism. As you will see, there are sometimes different possibilities for the synthesis of a target molecule. You will have to think critically of possibilities and then make a creative decision on the best route based on your knowledge of reactions.
As we have seen from the above example for the synthesis of the ether target molecule, there are two ways of making the ether bond. Alexander Williamson developed the Williamson reaction for the synthesis of ethers in 1850 and it involves an SN2 substitution reaction of an alkoxide anion and alkyl halide. As a result, the electrophile for the Williamson synthesis must be either primary or secondary and the nucleophile is made from a corresponding alcohol. An example of the Williamson synthesis of an ether is shown in Reaction (15-48).
(15-48)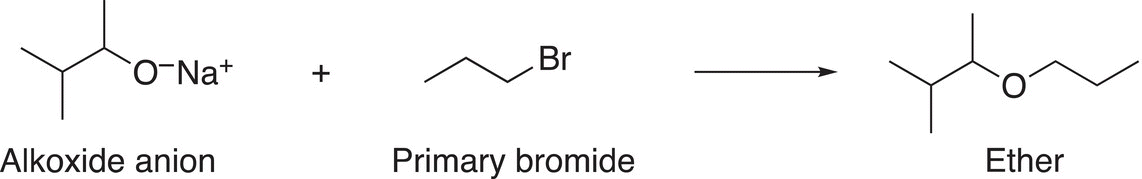
Problem 15.16
Utilize the Williamson synthesis to make the following compounds.
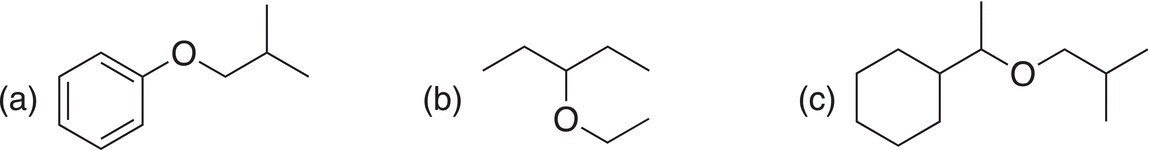
15.8.2 Synthesis of Nitriles
One of the most common methods of synthesizing nitriles involves a substitution reaction of the leaving group of an alkyl halide with a nitrile group. The synthesis of this bond is typically accomplished via a SN2 substitution reaction in a fairly polar nonprotic solvent as shown in Reaction (15-49).
(15-49)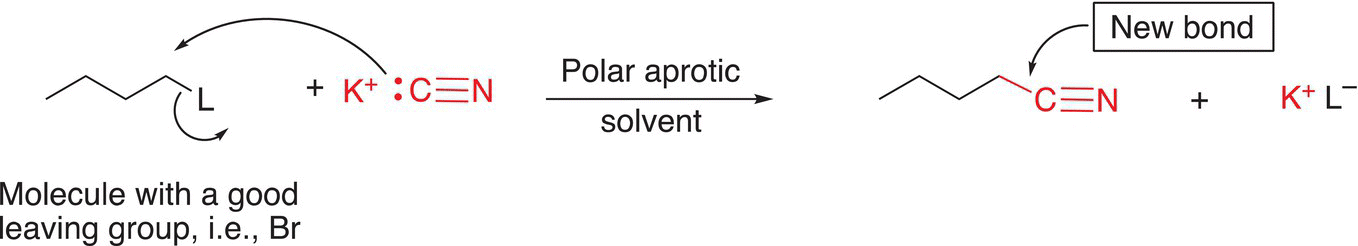
As we have seen, alkyl halides make good electrophiles and hence must be methyl, primary, or secondary, but never tertiary, if used for this type of synthesis. The two starting compounds that can be used for the synthesis of 3-methylpentanenitrile are shown in Reaction (15-50).
(15-50)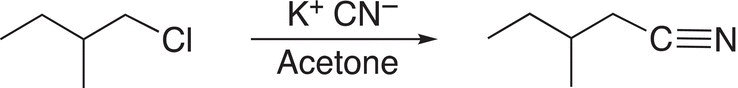
As pointed out, the reaction mechanism is an SN2 mechanism and, as a result, the electrophile must be methyl, primary or rarely secondary, but never tertiary.
Problem 15.17
Show how to synthesize the nitriles shown below in which substitution reactions are used.

15.8.3 Synthesis of Silyl Ethers
An important category of compounds in organic chemistry are silyl ethers, an example of a silyl is shown below.
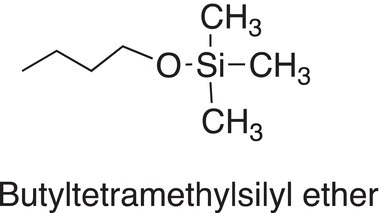
Its synthesis is shown below, and it involves the substitution of alkoxide group for a chloride anion leaving group, but most important, the reaction center is not a carbon as seen for most of the reactions thus far, its center is a silicon atom. As you know, silicon is just below carbon on the periodic table and hence its reactivity is very similar to that of carbon. Hence, the products of the reaction shown in Reaction (15-51) are not surprising and should be predictable based on utilizing the knowledge gained thus far.
(15-51)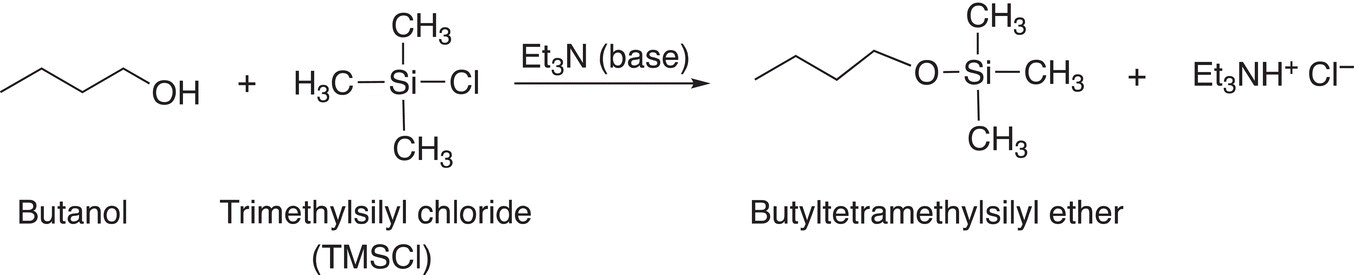
Problem 15.18
Give major organic products or starting alcohols to complete the reactions below.
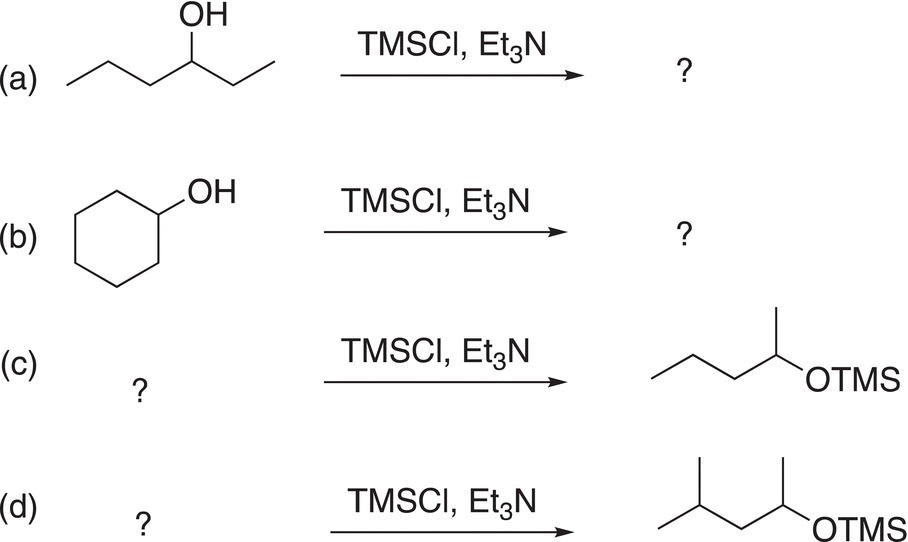
The TMS group can be used as a protecting group so that another reaction can take place at another functionality of a difunctional molecule. We have seen protecting groups before in the use of ethylene glycol to protect aldehydes and ketones when carrying out a reaction elsewhere in a molecule. In this case, the TMS can be used to protect an alcohol so that another reaction can take place elsewhere in a molecule. Consider the difunctional molecule, 5-bromopentanol, which contains a good leaving group in the form of a bromine and a nucleophilic alcohol functionality. Thus, a likely reaction for this molecule is the intramolecular substitution reaction shown in 15-52.
(15-52)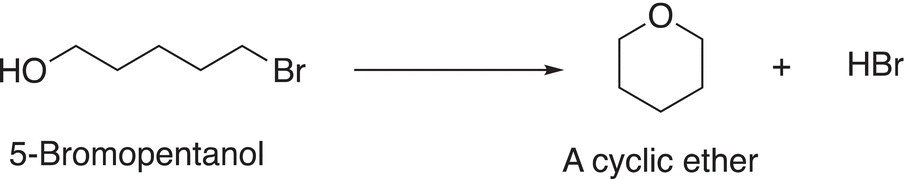
Another possible reaction that this molecule could be involved in is with another molecule depending on its concentration in the medium, as shown in Reaction (15-53).
(15-53)![]()
Thus, if we were trying to carry out the reaction shown in 15-54, the result will be a mixture of unwanted products.
(15-54)![]()
If you wanted to produce only the cyanide as the product and not the products like those obtained from Reactions (15-52) and (15-53), protection of the ─OH functionality is required so that the required reaction can take place at the other functionality. This strategy of protecting an alcohol functionality and then its removal to produce a target molecule by a multi-step synthesis is shown below.
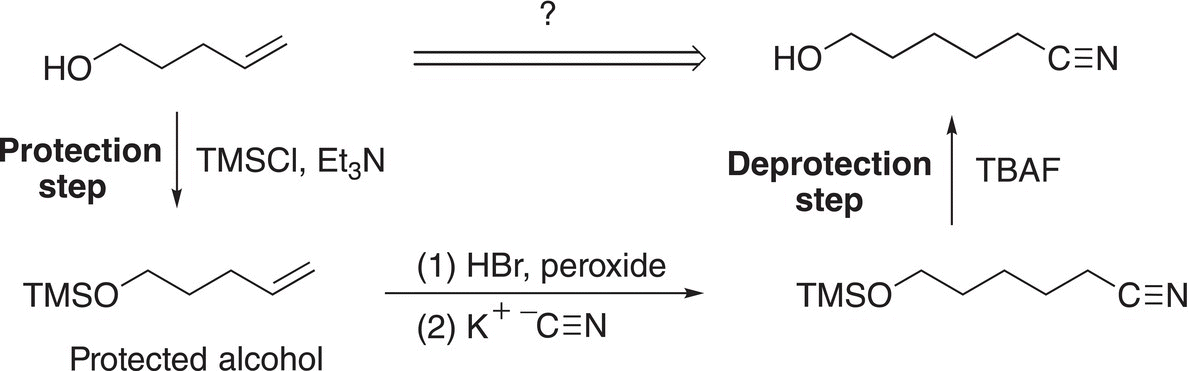
The protected alcohol is not a good nucleophile and hence will not react with the bromide functionality. As a result, the added reagent, the cyanide nucleophile, will displace the leaving group (the bromide anion) to form the product shown at the bottom of the scheme. To obtain the desired product, the TMS protection group must be removed and tetrabutyl ammonium fluoride (TBAF) is used to accomplish that task. The explanation of this reaction is shown in Reaction (15-55).
(15-55)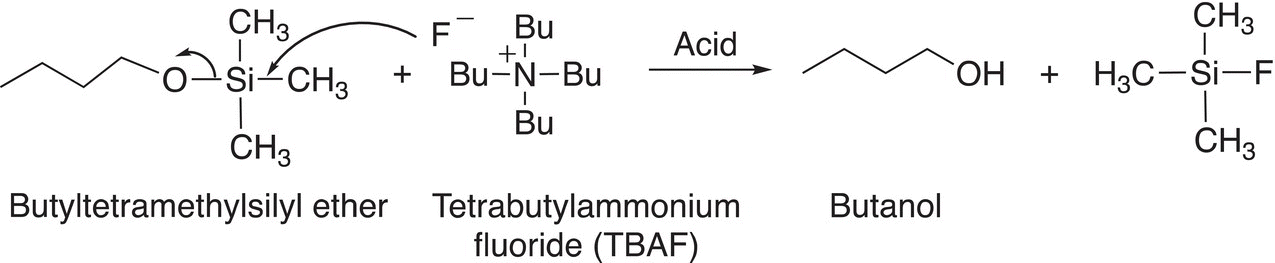
For the above reaction, the fluoride anion, being a good nucleophile, attacks the Si displacing the RO group, which is eventually protonated by the solvent or an acid in the reaction medium.
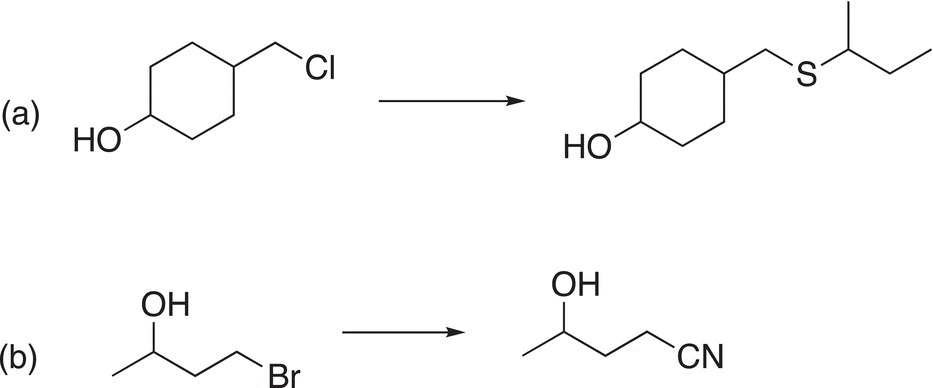
Problem 15.19
Show how to carry out the following transformations.
15.8.4 Synthesis of Alkynes
Alkynes that have the ability to form anions will react just as other nucleophiles described thus far. An example of the nucleophilic substitution reaction is shown in Reaction (15-56).
(15-56)
These reactions are SN2 reactions and care must be exercised in the selection of an appropriate solvent. The acetylide anion can be made easily by an appropriate acid—base reaction to remove the acidic proton of a terminal alkyne. Since the acetylide anion is a base, a protic solvent, such as water cannot be used as a solvent. Nonprotic solvents, such as acetone or DMSO, are typically good solvents for the synthesis of substituted alkynes by this route. An example for the synthesis of substituted alkyne is shown in Reaction (15-57).
(15-57)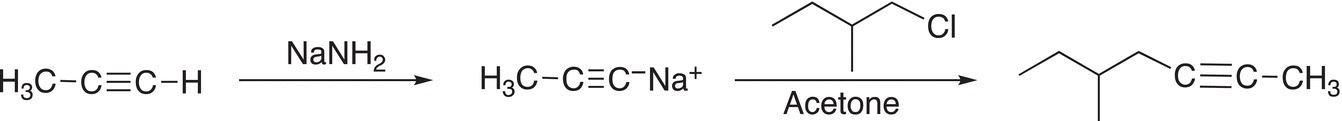
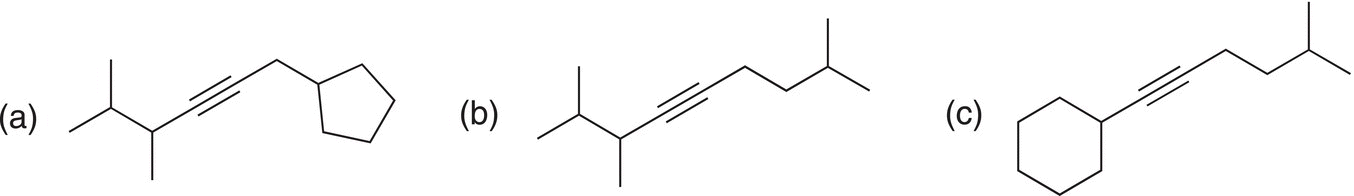
Problem 15.20
Starting with any terminal alkyne, show how to synthesize the following molecules.
15.8.5 Synthesis of α-Substituted Carbonyl Compounds
As we have seen in Chapter 7 on acids and bases, enolates are bases that are resonance stabilized and hence they are also of synthetic importance since they can also serve as nucleophiles for substitution reactions. Enolates react in a similar manner as any of the nucleophiles mentioned above, as shown in Reaction (15-58) in which there is a reaction with an enolate with an alkyl chloride to create a larger molecule with a new carbon—carbon bond in the α-position of the initial carbonyl compound.
(15-58)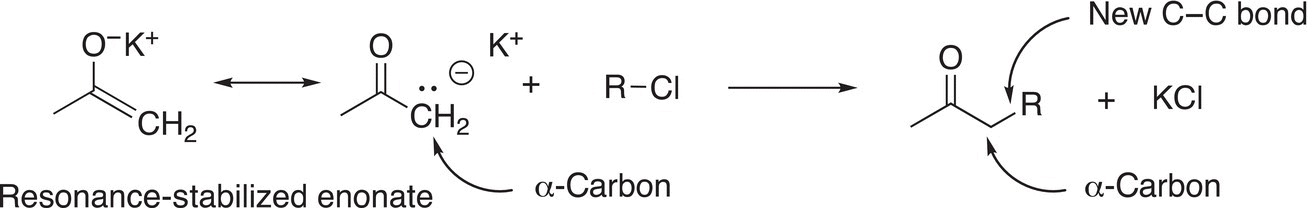
A specific application of this type of reaction in which a carbonyl compound is transformed into a larger α-substituted compound is shown below in Reaction (15-59).
(15-59)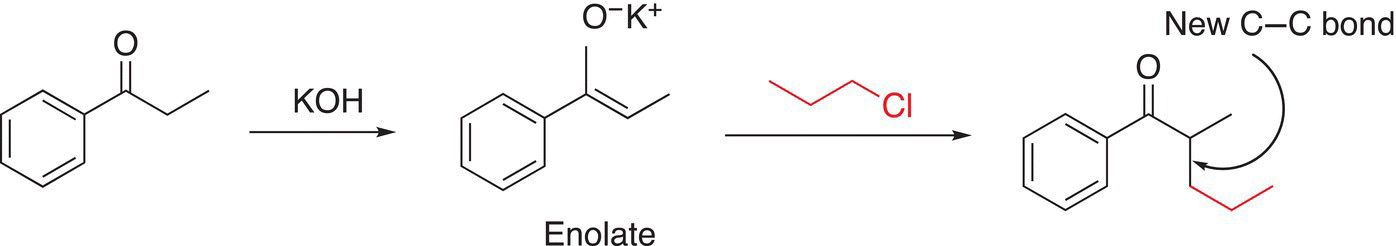
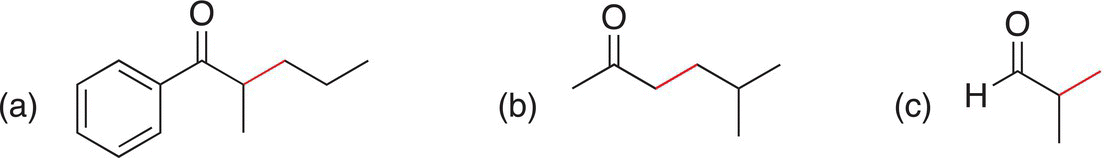
Problem 15.21
Starting with any carbonyl compound, show how to synthesize the following compounds. Consider making the bond in red.
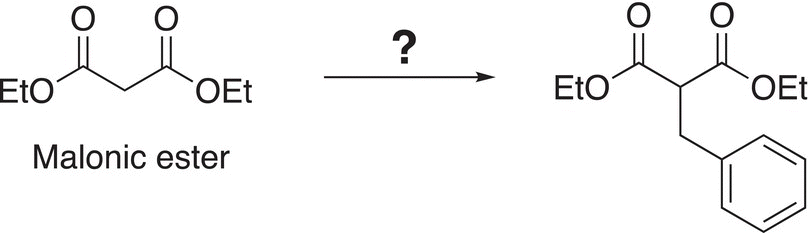
The stability of enolates can be increased if the negative charge of the conjugate enolate is delocalized over a larger number of atoms. Diethyl malonate (malonic ester) is one such example and it is a fairly acidic molecule. These compounds are of importance in the synthesis of substituted acetic acids. Shown in Reaction (15-60) is an example of a reaction in which malonic ester is used as a nucleophile after deprotonation to synthesize a larger molecule.
(15-60)α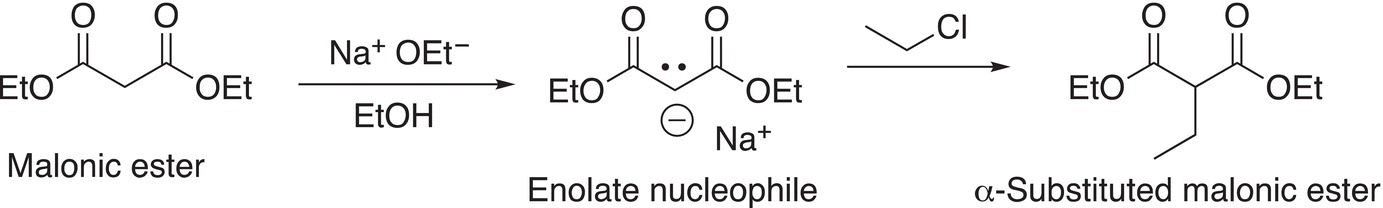
Note that the α-substituted malonic ester that is produced by this reaction has an α-hydrogen that can be abstracted to form a new nucleophile, which can undergo a similar reaction as shown in Reaction (15-61) to make an even larger and more complex molecule.
(15-61)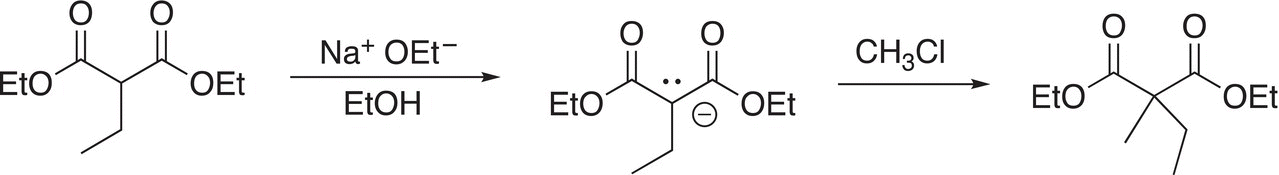
In later chapters, we will learn further transformations that these molecules can undergo to eventually synthesize molecules as the target molecule shown in Reaction (15-62).
(15-62)
Problem 15.22
Show how to carry out the following transformation, clearly show all reagents in your reaction sequence
In summary, when designing a synthetic route for a target molecule, you must first identify the functional group in the target molecule and determine the bond of the functional group that must be made. Once you have made that identification, you should next devise a route to make that bond using an appropriate electrophile and also an appropriate nucleophile. Finally, review your synthesis to make sure that you have made a strategic choice. That is, if you want the reaction to proceed via an SN1 route, then you must make sure of appropriate solvent and type of electrophile. If you want the reaction to proceed via an SN2 route based on perhaps a required stereochemistry, then you will have to make sure that the solvent is nonionizing, the nucleophile is small and not very basic and the electrophile is primary or secondary, but never tertiary.