Organic Chemistry: Concepts and Applications - Headley Allan D. 2020
Nucleophilic Substitution Reactions at Acyl Carbons
16.5 Substitution Reactions Involving Esters
For substitution reactions involving esters, the leaving group is not as a good leaving group as those that we have seen thus far in this chapter, which are the chloride anion and the carboxylate anion. For reactions involving esters, the leaving group is an alkoxide anion. As we have demonstrated in Chapter 7 when we looked at acids and bases, we saw that the conjugate acid of the alkoxide anion is an alcohol. Alcohols have pKa values of approximately 20, which means that they are very weak acids, and as a result, the conjugate bases, alkoxide anions, are extremely strong bases and hence a very poor leaving groups as shown in Reaction (16-46).
(16-46)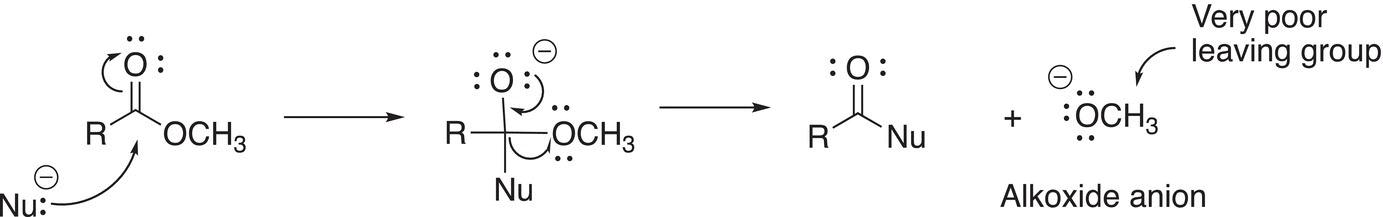
In an acidic medium, however, the alkoxide ion is converted into an extremely good leaving group, a protonated alcohol. Thus, the hydrolysis of esters is typically acid catalyzed. As you will see from the mechanism, however, the alkoxide leaving group is not first protonated in order to make it a good leaving group. Let us look at the mechanism a bit closer. In the first step of the mechanism, the proton protonates the most basic site of the ester molecule, as shown in Reaction (16-49).
(16-47)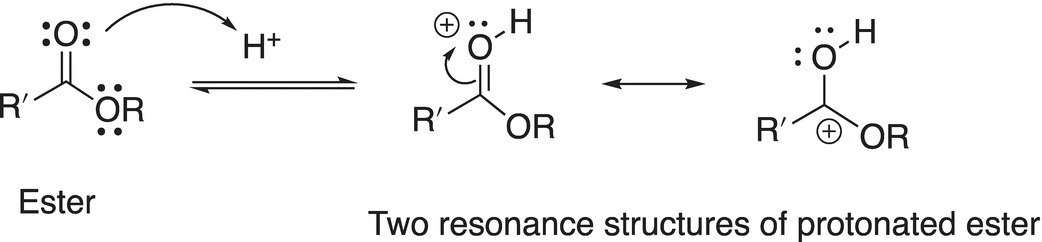
Note that the most basic site of the ester molecule is the lone pair of electrons on the carbonyl oxygen and not the lone pair of electrons on the alkoxide oxygen as demonstrated by the stable resonance structure that results upon protonation at the carbonyl oxygen site. In another step, the nucleophile attacks the carbonyl carbon to form a tetrahedral intermediate as shown in Reaction (16-48).
(16-48)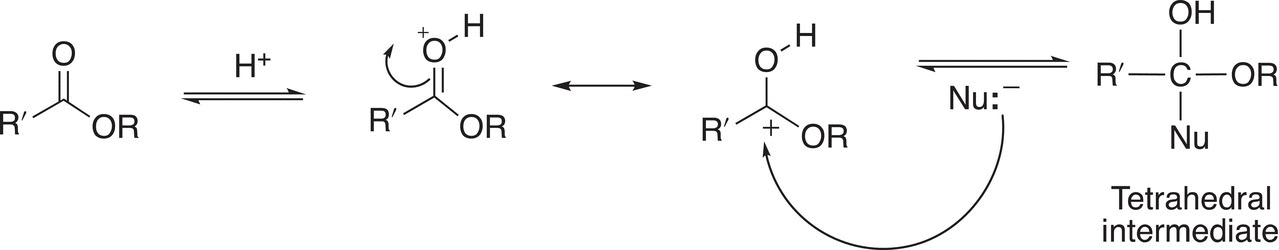
As pointed out previously, the stability of the tetrahedral intermediate depends on the size of the R group that is bonded to the carbonyl carbon. Very crowded tetrahedral intermediates have bond angles that are strained and are less than the desired 109.5° and, as a result, less stable than tetrahedral intermediates with smaller and less bulky alkyl groups bonded to the carbonyl carbon. In the presence of an acid, protonation of the OR will occur and as we have seen earlier, this protonation transforms the OR group into a good leaving group as shown in Reaction (16-49).
(16-49)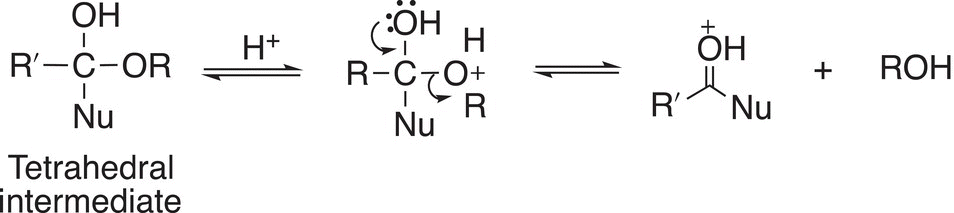
In a final equilibrium step of the mechanism, the proton is lost to generate the organic product as shown in Reaction (16-50).
(16-50)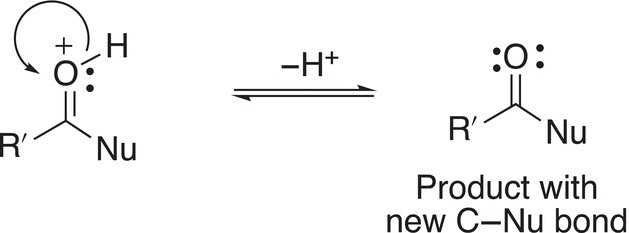
This step demonstrates that the proton is really a catalyst since it was used in the first step and regenerated in this step. It is consumed in the first step of the mechanism and liberated in the last step of the mechanism.
Thus, even though this leaving group of esters is not a very good leaving group, compared to those of the acid chlorides and anhydrides, under acidic conditions, it is converted to a very good leaving group, ROH.
16.5.1 Substitution Reactions of Esters with Water
The reaction of esters with water is very similar to that of the hydrolysis of the previous acyl compounds that we have examined earlier, except that the leaving group for the ester is the alkoxide anion, which is a poor leaving group. In the presence of a basic aqueous medium and at elevated temperatures, hydrolysis of esters can still occur, despite having a very poor leaving group. The first step of the reaction of methyl propanoate under basic conditions is shown in Reaction (16-51).
(16-51)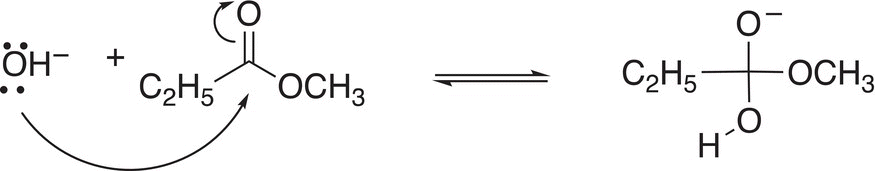
In the next step of the mechanism, the alkoxide anion, which is a very poor leaving group leaves as shown in Reaction (16-52).
(16-52)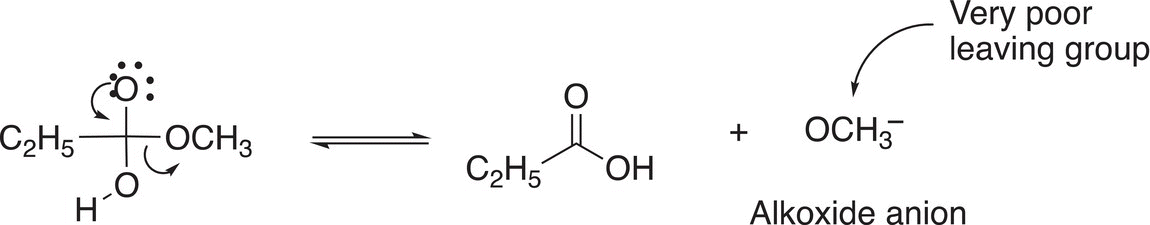
Since the alkoxide anion is a very strong base, an acid—base reaction will occur involving the carboxylic acid to give the carboxylate salt and an alcohol, as shown in Reaction (16-53).
(16-53)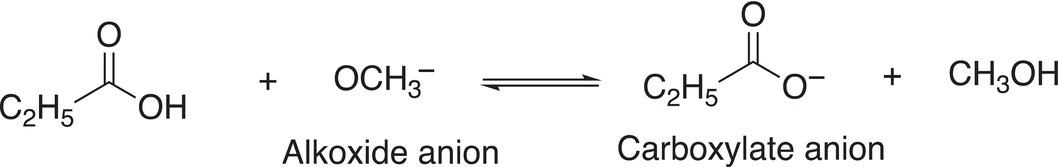
The basic hydrolysis of esters is also called saponification and this type of reaction is used in the production of soap. As pointed out earlier, these reactions are carried out at elevated temperatures. If the hydrolysis is carried out using the esters of fatty acid and lye (a base), the carboxylate salt that is produced is used as soap. In Chapter 20, a more detailed discussion of the type of esters that are used to produce soaps and how soaps work will be discussed. The saponification of a triglyceride (tri-ester) is shown in Reaction (16-53).
(16-54)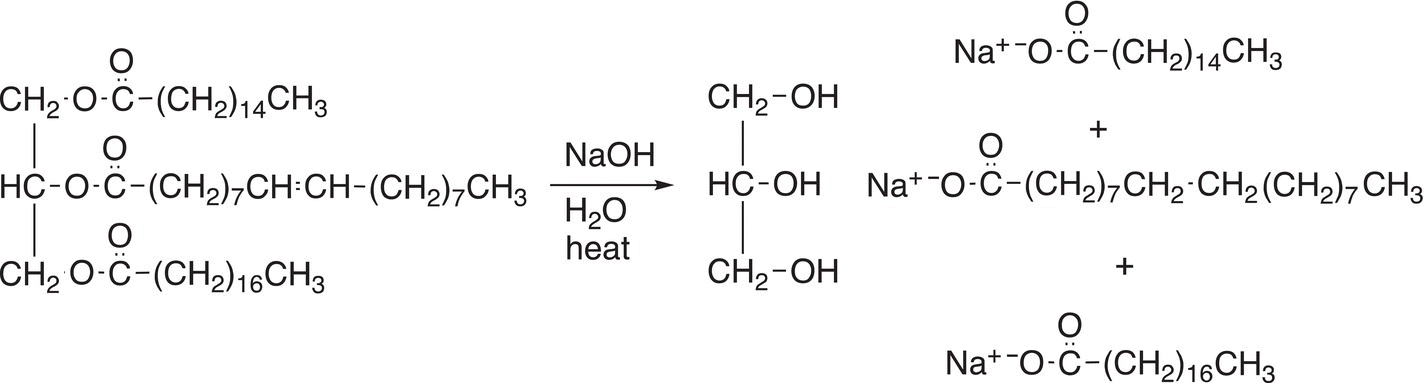
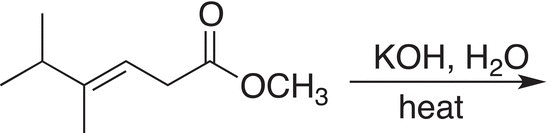
Problem 16.18
Give the products that result from the basic hydrolysis of the compound shown below.
Hydrolysis of esters carried out under acidic conditions promotes the departure of a better leaving group as an alcohol. As a result, an acid-catalyzed reaction will occur, the mechanism for the hydrolysis of methyl propanoate is shown in Reactions (16-55) and (16-56).
(16-55)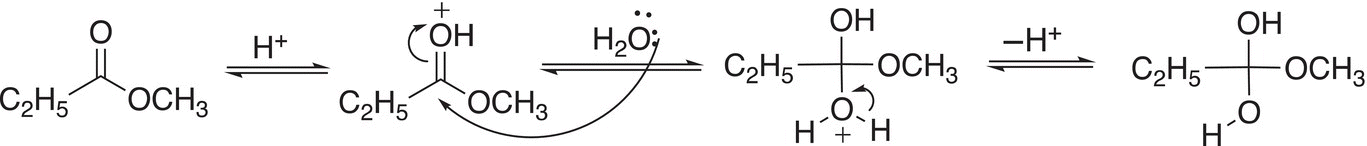
(16-56)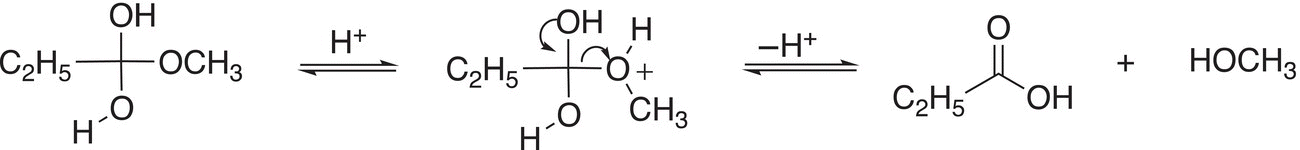
16.5.2 Substitution Reactions of Esters with Alcohols
The reaction of esters with alcohols is also called transesterification since another ester is produced as a product. As you can imagine, the reaction is very similar to that of the reaction of water with esters and an example of a transesterification is shown in Reaction (16-57).
(16-57)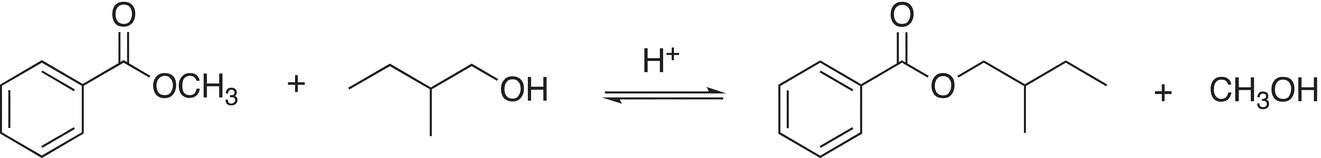
Note that these reactions are shown as equilibrium reactions, and the equilibrium can be manipulated as you have seen in general chemistry via Le Chatelier's Principle. By removing the methanol in the product or by having a large concentration of the reactant alcohol, it is possible to shift the equilibrium to the right in order to produce the ester shown in the product. On the other hand, if the concentration of the methanol is high, the equilibrium will favor the reverse direction.
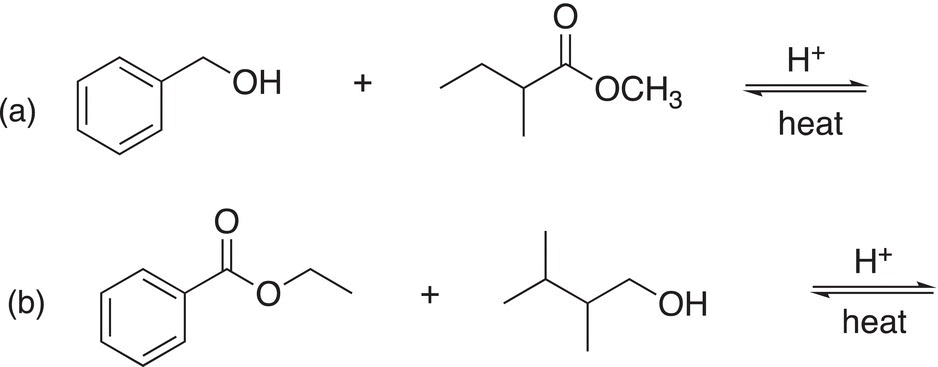
Problem 16.19
Give the organic products of the following transesterification reactions.
Intramolecular reactions involving difunctional molecules with an alcohol and ester functionalities are possible and produce cyclic esters, also called lactones as products. The general reaction is given in Reaction (16-58).
(16-58)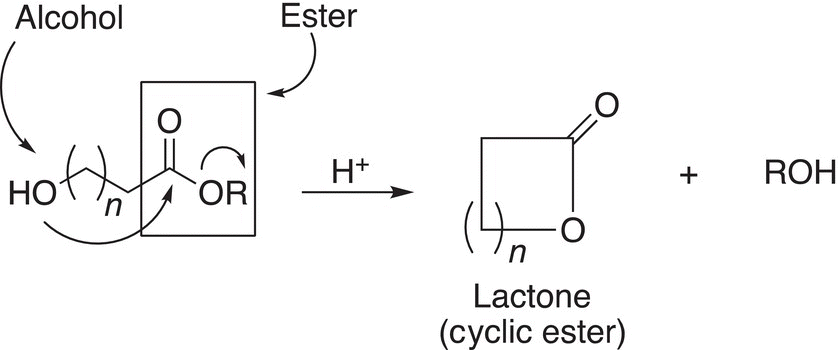
Reaction (16-59) gives an example of a transesterification reaction to form a six-member ring cyclic ester (lactone).
(16-59)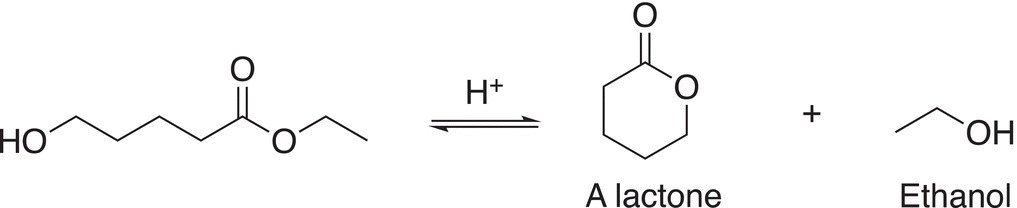
16.5.3 Substitution Reactions of Esters with Ammonia and Amines
Let us now examine the substitution reaction of esters with ammonia and amines. The mechanism for these reactions is very similar to those of acid chlorides, except as mentioned, an acidic catalyst must be used and these reactions are carried out at elevated temperatures. Since ammonia and amines are nucleophilic molecules, they will react with ester to form the corresponding primary amide (with ammonia); secondary amide (with primary amines); and tertiary amide (with secondary amines). The other product that is produced is an alcohol.
Reaction (16-60) gives the reaction of an ester with ammonia.
(16-60)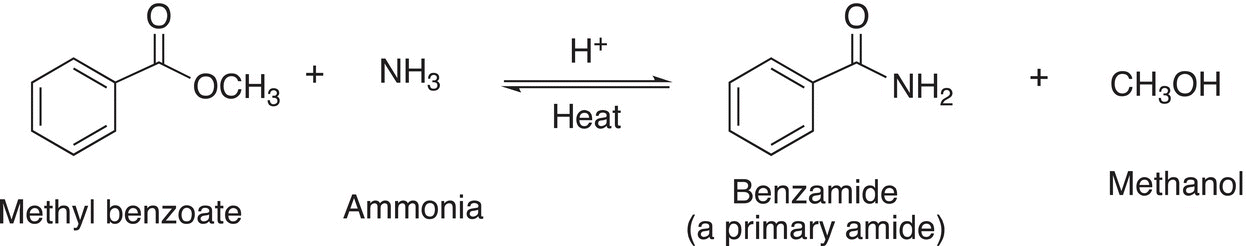
Reaction (16-61) shows the reaction of an ester with a primary amine, methylamine.
(16-61)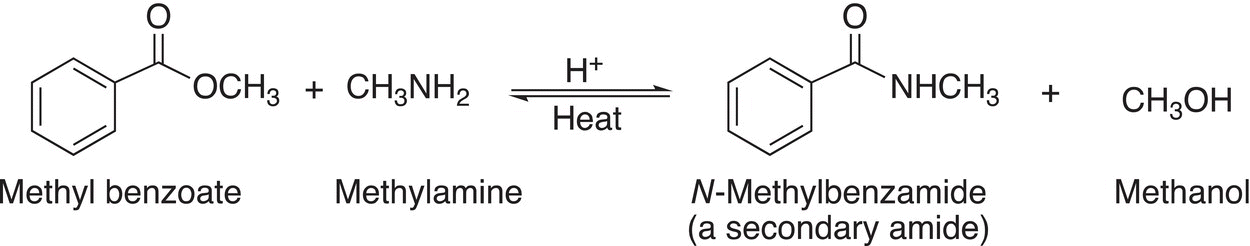
Reaction (16-62) gives the reaction of an ester with a secondary amine, dimethylamine.
(16-62)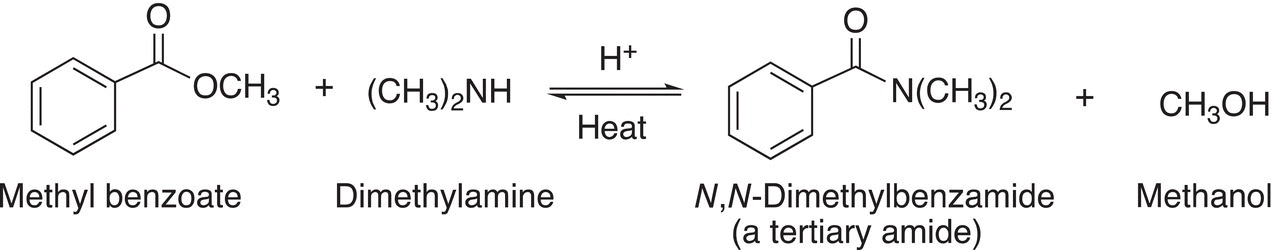
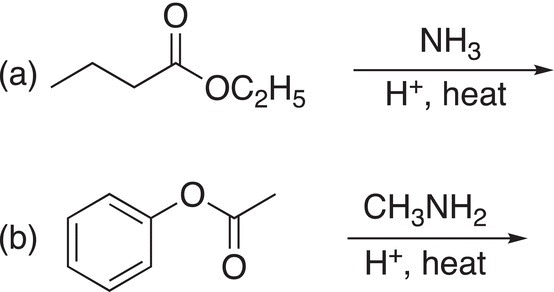
Problem 16.20
Give the products of the following reactions.
16.5.4 Substitution Reactions of Esters with Soft Organometallic Reagents
As you might predict, the reactions of organometallic reagents with esters are similar to those of acid chlorides. Owing to the reactivity of organometallic reagents, a catalyst is not required for these reactions. Substitution reactions involving different organocuprates and esters are shown in Reactions (16-63) and (16-64).
(16-63)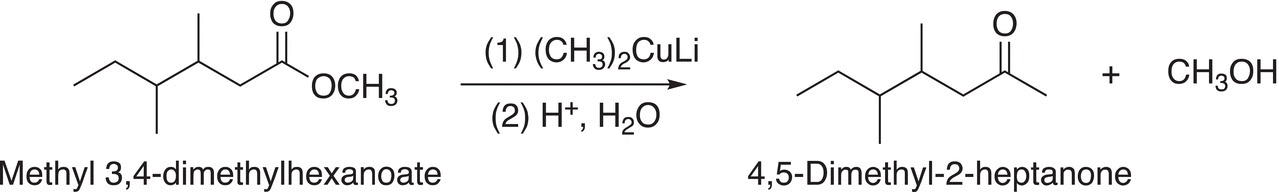
(16-64)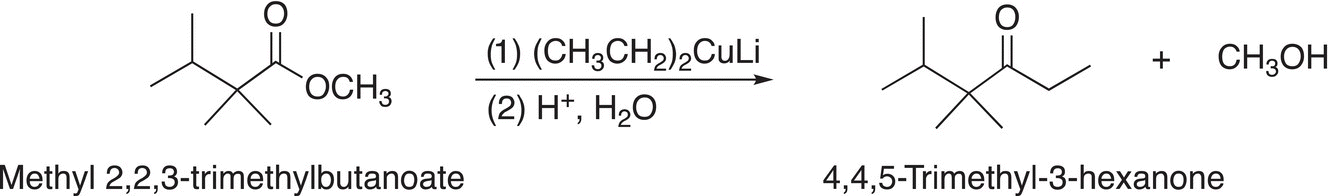
16.5.5 Substitution Reactions of Esters with Hard Organometallic Reagents
As mentioned several times earlier, Grignard reagents in addition to being very strong bases are also good reducing agents, and they will react with esters to eventually form the corresponding alcohol after hydrolysis in the presence of excess Grignard reagents. The reactions shown in Reactions (16-65) and (16-66) are examples of Grignard reagents reacting with esters to produce alcohols after hydrolysis.
(16-65)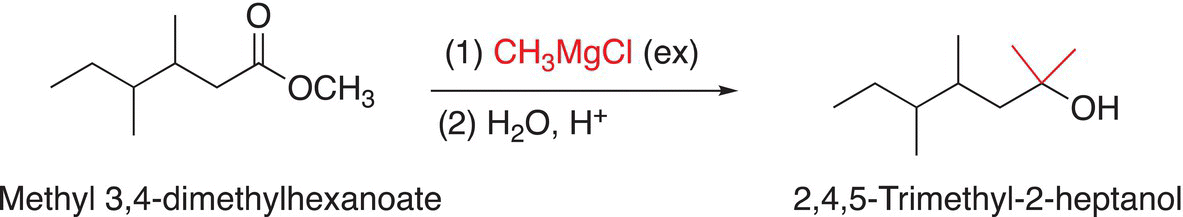
(16-66)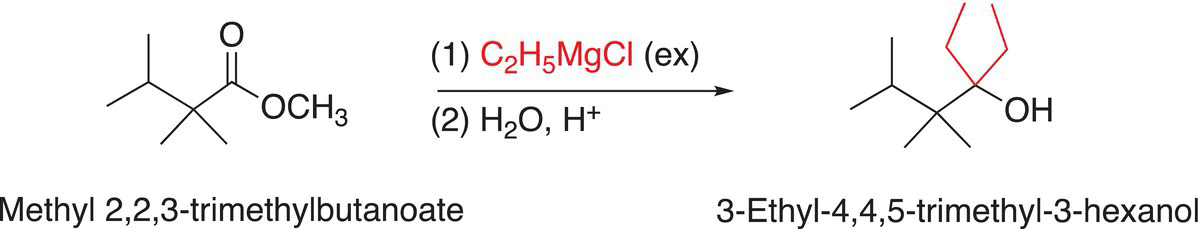
Note that one of the outcomes for these reactions is that esters are transformed into tertiary alcohols, which contains two of the same alkyl groups that came from the Grignard reagent. These groups are shown in red in Reactions (16-65) and (16-66).
Problem 16.21
Show how to synthesize each of the following alcohols by using an ester and a Grignard Reagent.
Hint: identify the two groups that are the same, they are from the Grignard reagent.
16.5.6 Substitution Reactions of Esters with Soft and Hard Metallic Hydrides
The reactions of esters with metal hydrides, such as tri-tert-butoxyaluminum hydride, result in aldehydes. Reactions shown in (16-67) and (16-68) are examples of the reaction of esters with tri-tert-butoxyaluminum hydride to produce aldehydes.
(16-67)
(16-68)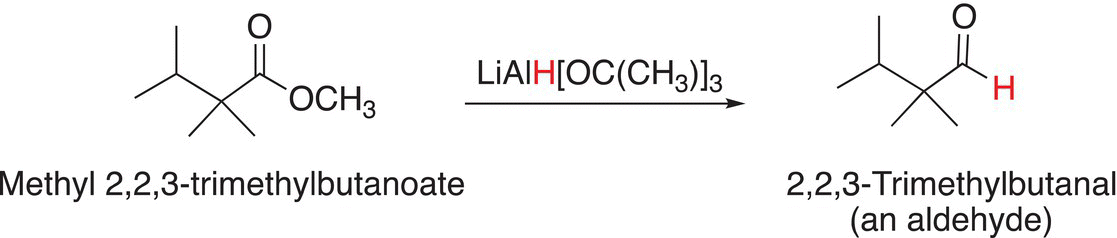
On the other hand, in the reaction with a much stronger nucleophilic reducing agent, such as LAH, the corresponding alcohol will be the product as shown Reactions (16-69) and (16-70).
(16-69)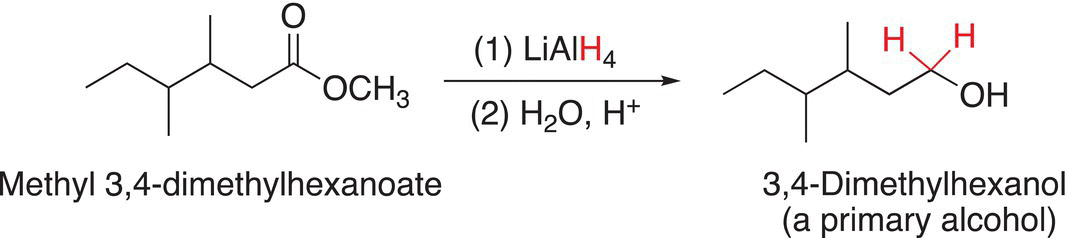
(16-70)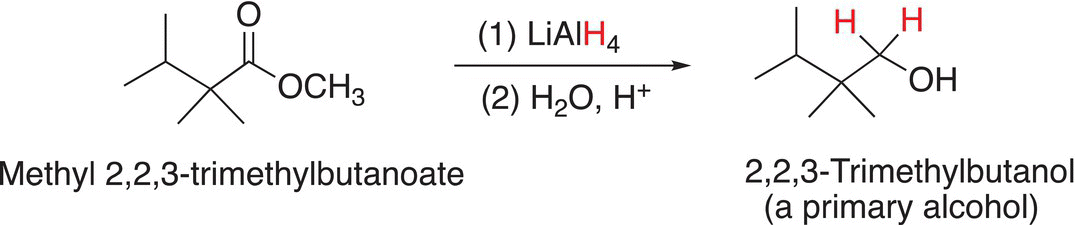
Problem 16.22
Show the structures of appropriate esters that can be used as starting compounds for the synthesis of the molecules shown below. Utilize LiAlH4 as a reducing agent, followed by acidic workup to obtain the final product.
16.5.7 Substitution Reactions of Esters with Enolates of Esters
Enolates of esters are formed by the deprotonation of an ester that contain at least one α-hydrogen, as shown in Reaction (16-71).
(16-71)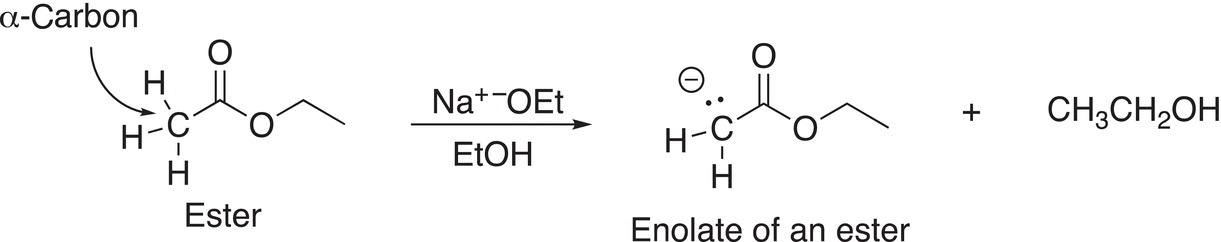
The reactions that enolates of esters undergo are very similar to the substitution reactions of enolates that were discussed in Chapter 15, except in this case, the enolate can react with another mole of an ester by an acyl substitution reaction as shown in Reaction (16-72) to produce a β-keto ester.
(16-72)β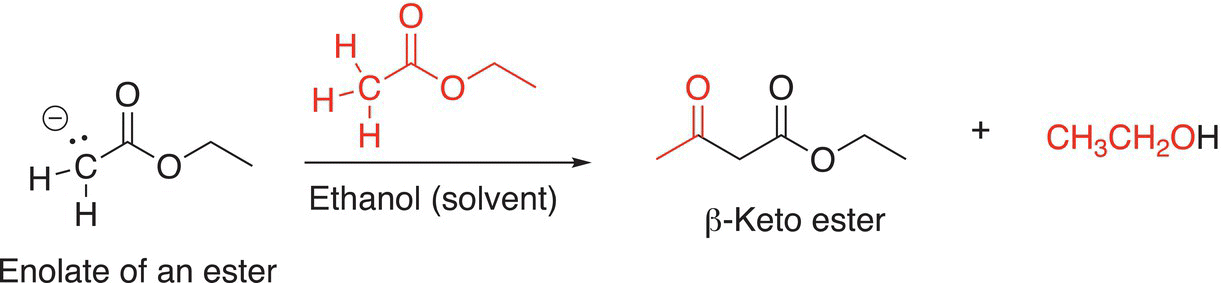
This type of acyl substitution condensation reaction is known as a Claisen condensation reaction. As you can imagine, it is possible for an intramolecular Claisen reaction to occur as shown in Reaction (16-73).
(16-73)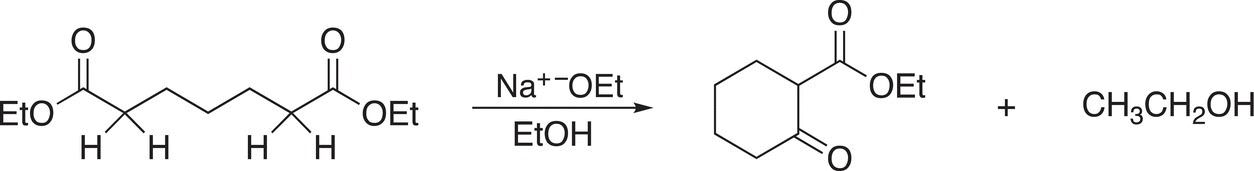
In the first step of the mechanism, the ethoxide anion base abstracts a proton from the alpha carbon to form an enolate anion, which then attacks the carbonyl carbon of the ester as shown in Reaction (16-74) to form a tetrahedral intermediate.
(16-74)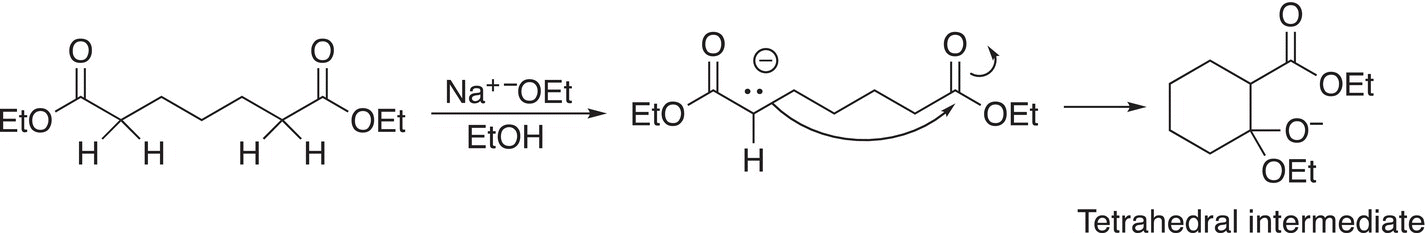
In the next step of the mechanism, the tetrahedral intermediate forms the carbonyl compound with the ethoxide anion being protonated by the solvent to form ethanol as the other product as shown in Reaction (16-75).
(16-75)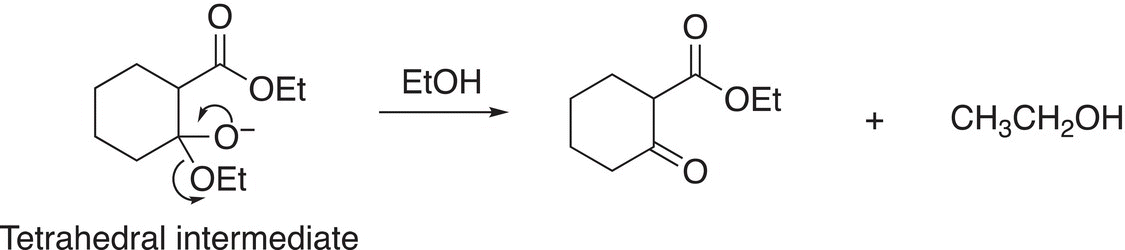
This type of intramolecular Claisen condensation is known as the Dieckmann reaction. Another example of the Dieckmann reaction is shown in Reaction (16-76).
(16-76)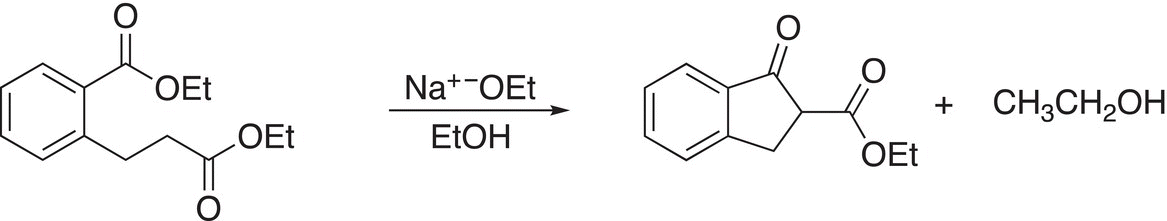
As you can imagine, it is possible for enolates of esters that have α-hydrogen(s) to react with esters that do not have α-hydrogen(s) as shown in the reaction in Reaction (16-77).
(16-77)αβ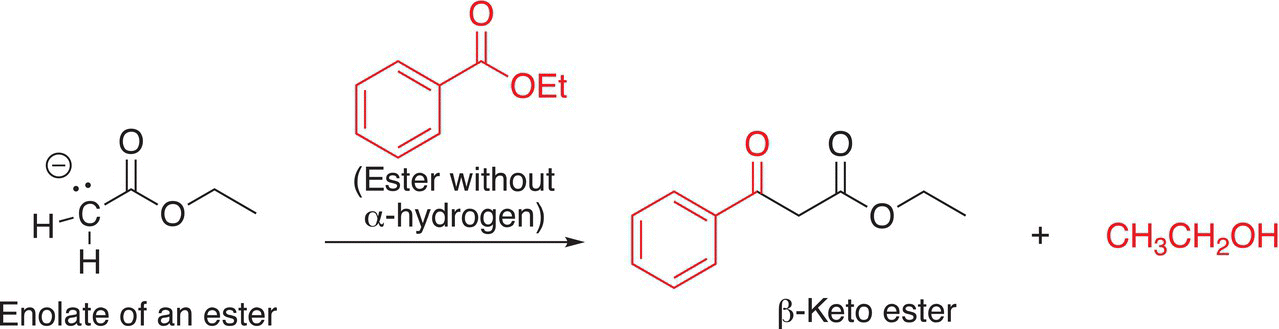
These types of reactions are known as crossed Claisen condensation reactions.
Since the compounds produced from these reactions are esters, they can be hydrolyzed in the presence of acid and water to form carboxylic acids as shown in the example given in Reaction (16-78).
(16-78)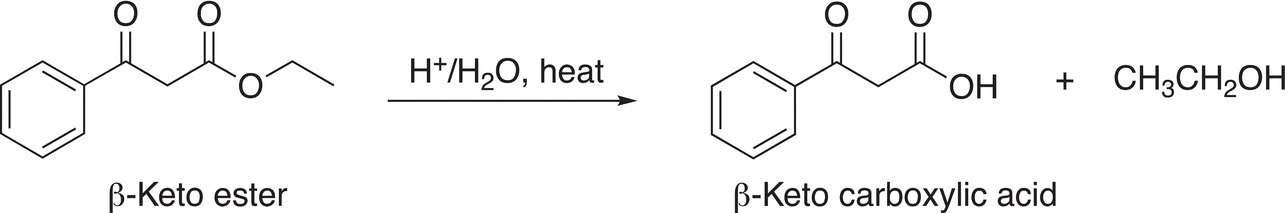
In the presence of heat, β-keto carboxylic acids readily decompose to give carbon dioxide and a ketone as shown in the example given in Reaction (16-79).
(16-79)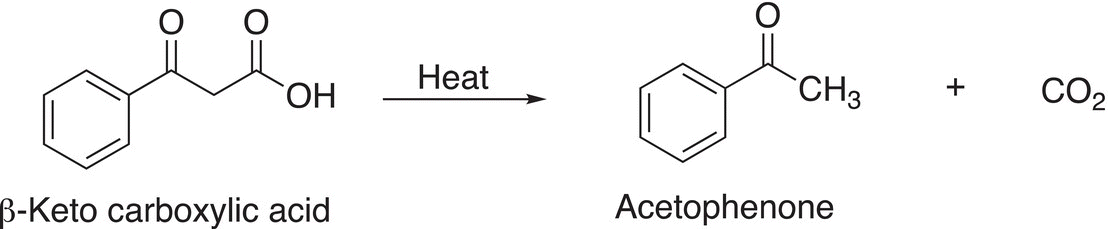
The loss of carbon dioxide from a β-keto carboxylic acid results in the formation of an enol as shown in the mechanism given in Reaction (16-80).
(16-80)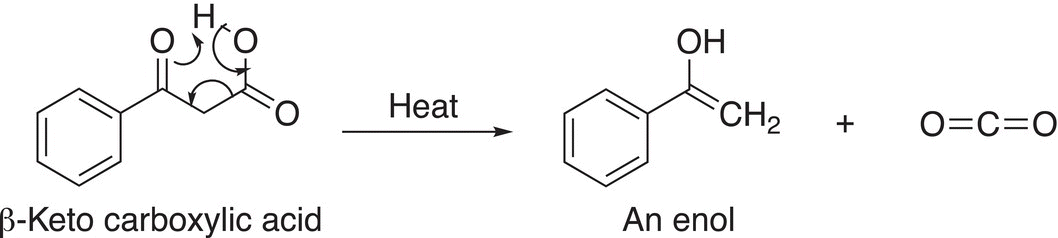
As we have seen previously, enols readily tautomerize to give the more stable keto form, as shown in the example given in Reaction (16-81).
(16-81)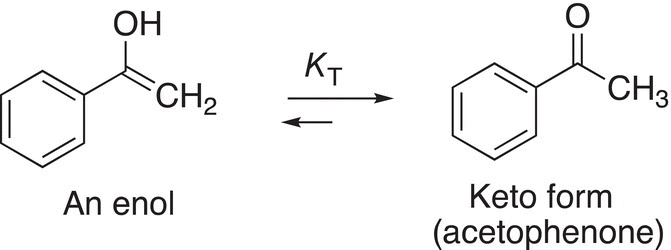
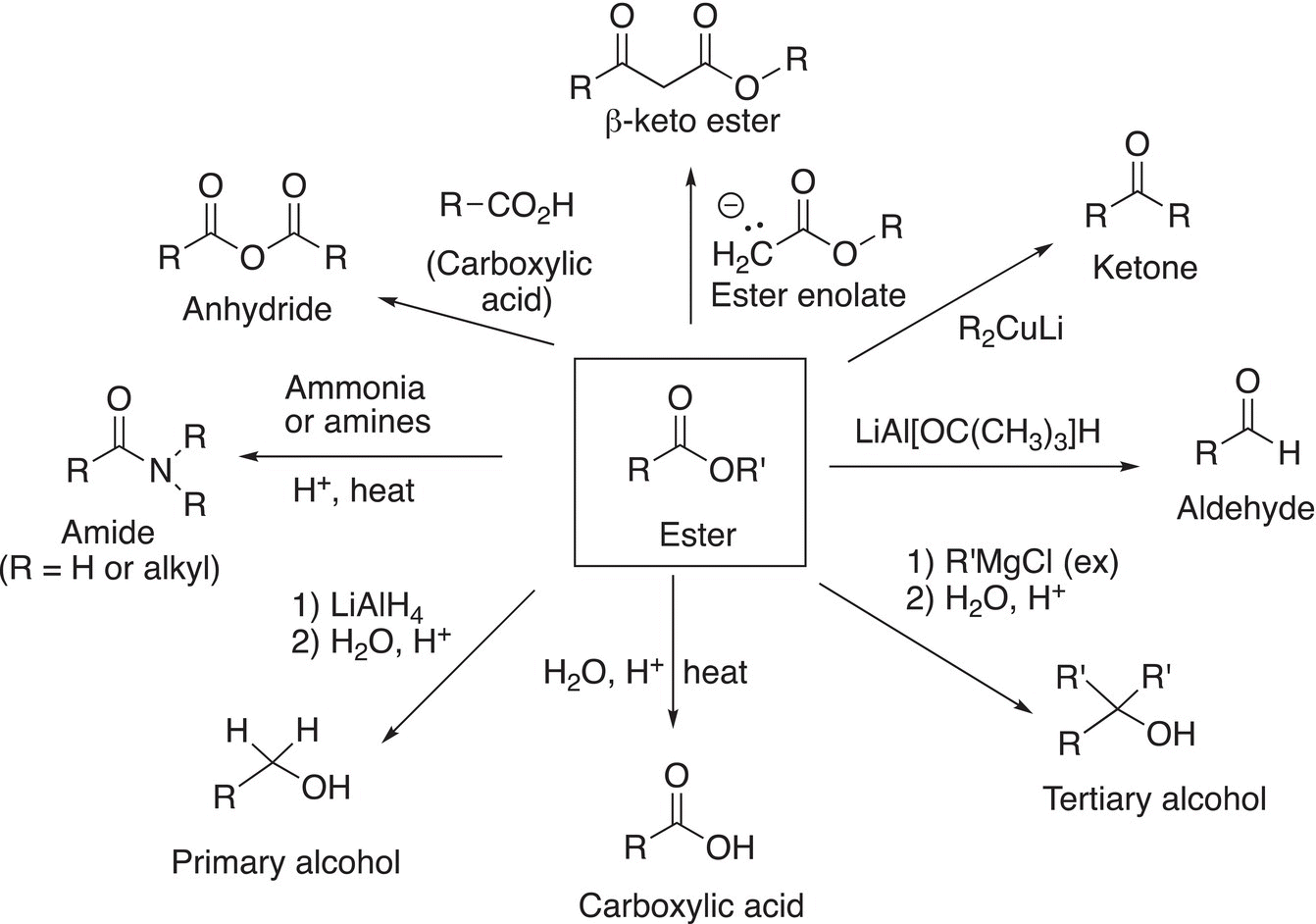
Figure 16.2 Summary of the reactions of esters.
Shown in Figure 16.2 is a summary of reactions that esters undergo.