Organic Chemistry: Concepts and Applications - Headley Allan D. 2020
Nucleophilic Substitution Reactions at Acyl Carbons
16.9 Substitution Reactions Involving Sulfur Containing Compounds
Owing to the similarity of the carbon oxygen double bond with the sulfur oxygen double bond, we will examine substitution reactions involving thionyl chloride and phenyl sulfonyl chloride. Interestingly, these reactions are used to convert the poor leaving ─OH group to a better leaving group, the chloride and the tosylate, respectively. Reaction (16-105) gives an example of the reaction of thionyl chloride with an optically active alcohol.
(16-105)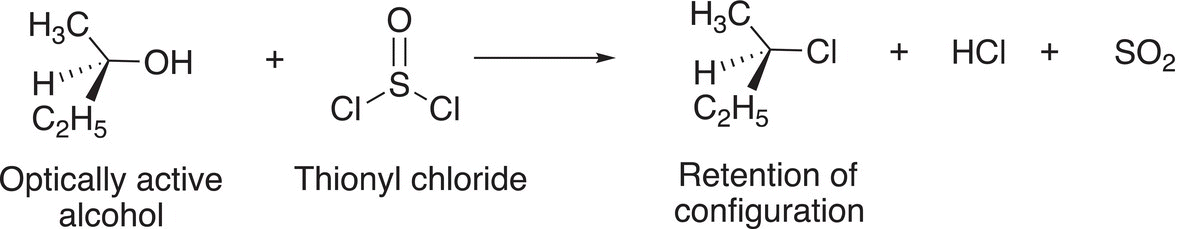
These reactions result in retention of configuration if the reactant is an optically active alcohol. The mechanism given below explains this outcome. In the first step, the nucleophilic alcohol attacks the electrophilic sulfur atom and a chloride ion is eventually displaced as shown in Reaction (16-106)
(16-106)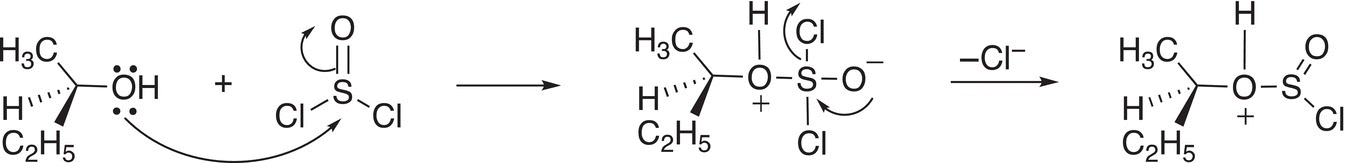
In another step, the chloride anion abstracts a proton to form HCl and the resulting intermediate breaks apart liberating sulfur dioxide (SO2) an excellent leaving group, which leaves as a gas. Simultaneously, the other chloride anion leaves then bonds to the carbon from the same side, hence the retention of configuration as shown in Reaction (16-107).
(16-107)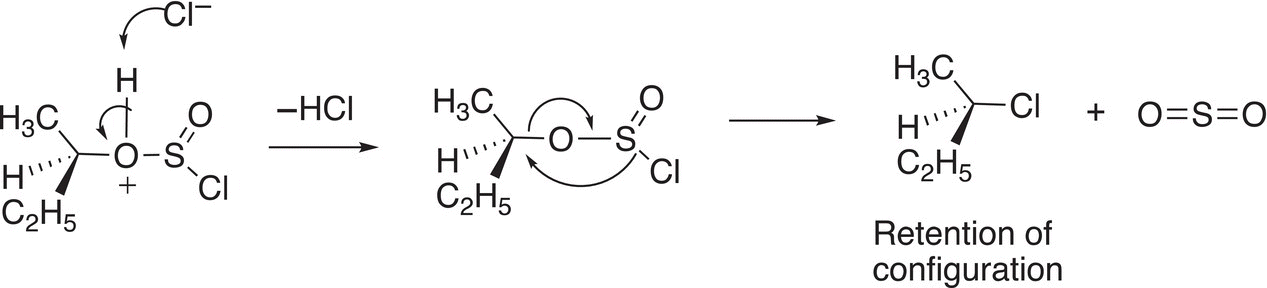
Note in the last step of the mechanism, there is an internal SN2 attack of the chloride anion resulting in a substitution. Since this step involves an SN2 attack, these reactions work best for primary or secondary alcohols.
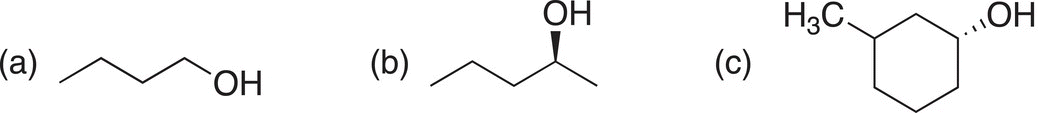
Problem 16.28
Give the products of the reaction of each of the compounds shown below with SOCl2, give stereochemistry where appropriate.
Another sulfur reagent that can be involved in similar substitution reactions is p-toluenesulfonyl chloride, also known as tosyl chloride (TsCl). The general reaction of TsCl with a nucleophile is shown in Reaction (16-108).
(16-108)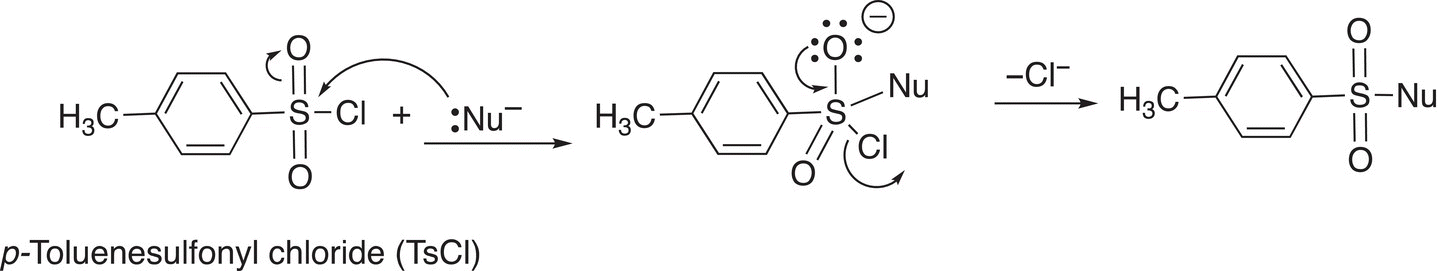
As you can imagine, the sulfur atom is very electrophilic since it is bonded to two electronegative oxygen atoms and also to a chlorine, which is a good leaving group. A specific example of this substitution reaction is given in Reaction (16-109).
(16-109)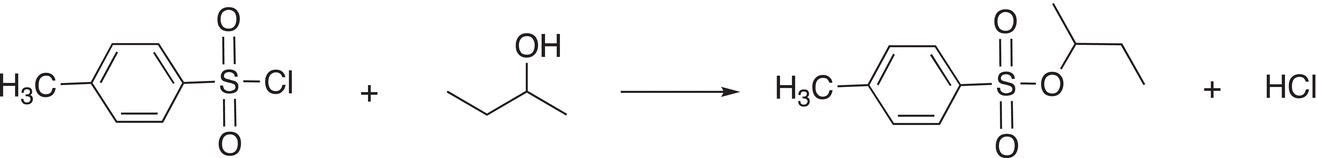
An advantage of reactions of this type is that the very poor ─OH leaving of alcohols is essentially converted to an extremely good leaving group. Reactions of this type give another option of converting the ─OH of alcohols to a good leaving group as demonstrated in the reaction in Reaction (16-110).
(16-110)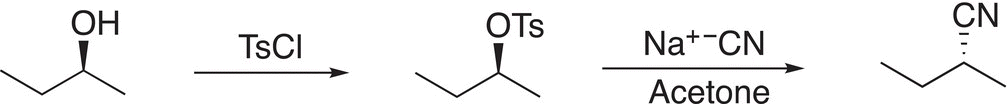
For this above reaction, note that the stereochemistry of the alcohol reactant is retained for the first reaction. Since the second reaction in the given sequence of reactions is SN2 (as indicated by the solvent), there is an inversion of stereochemistry in the product.