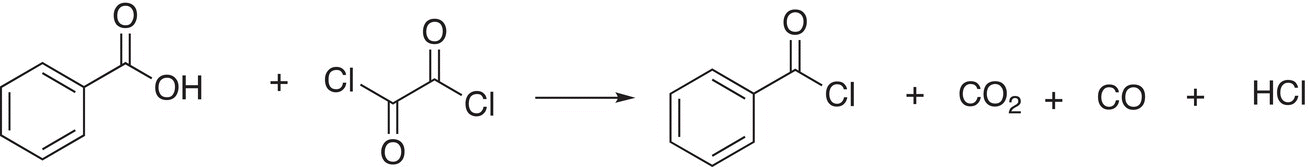
Organic Chemistry: Concepts and Applications - Headley Allan D. 2020
Nucleophilic Substitution Reactions at Acyl Carbons
End of Chapter Problems
1. 16.31 Show how the following compounds can be prepared from benzoyl chloride.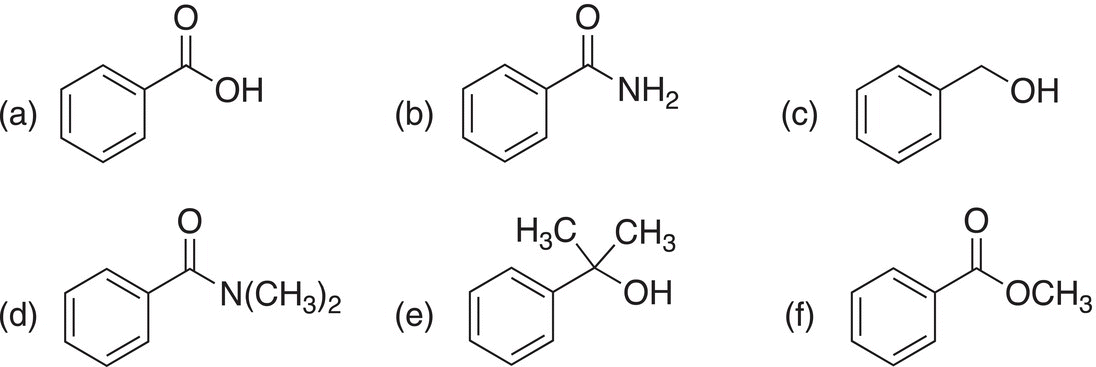
2. 16.32 Give the structural formulas for the final major organic products for each of the following reactions or sequence of reactions.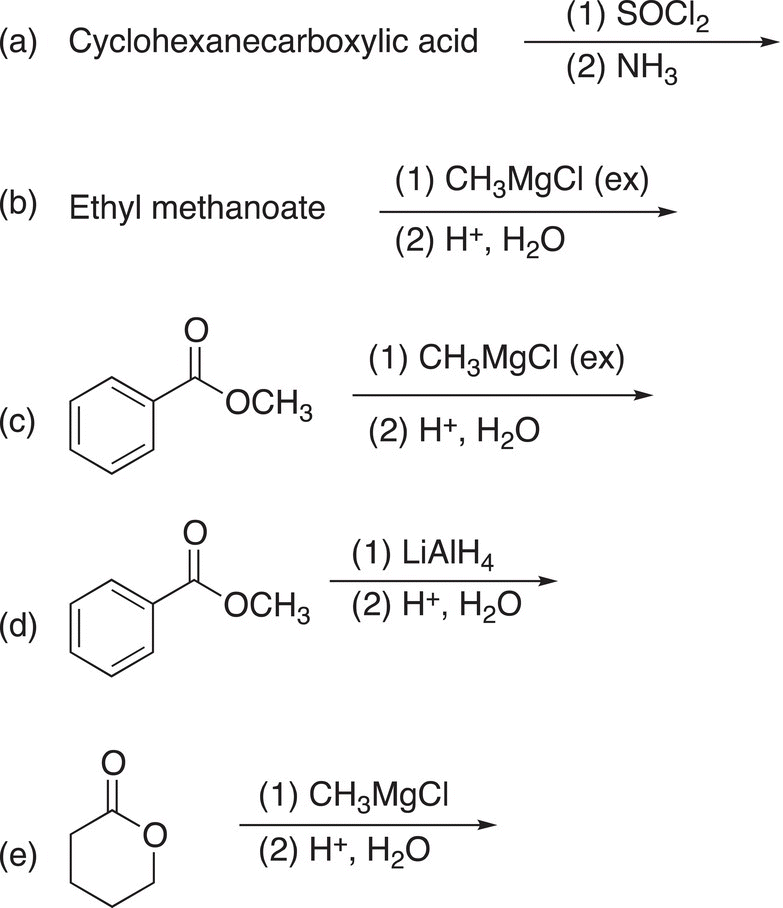
3. 16.33 Show how to carry out the following transformations. Clearly indicate all reagents and reaction conditions used in your synthesis.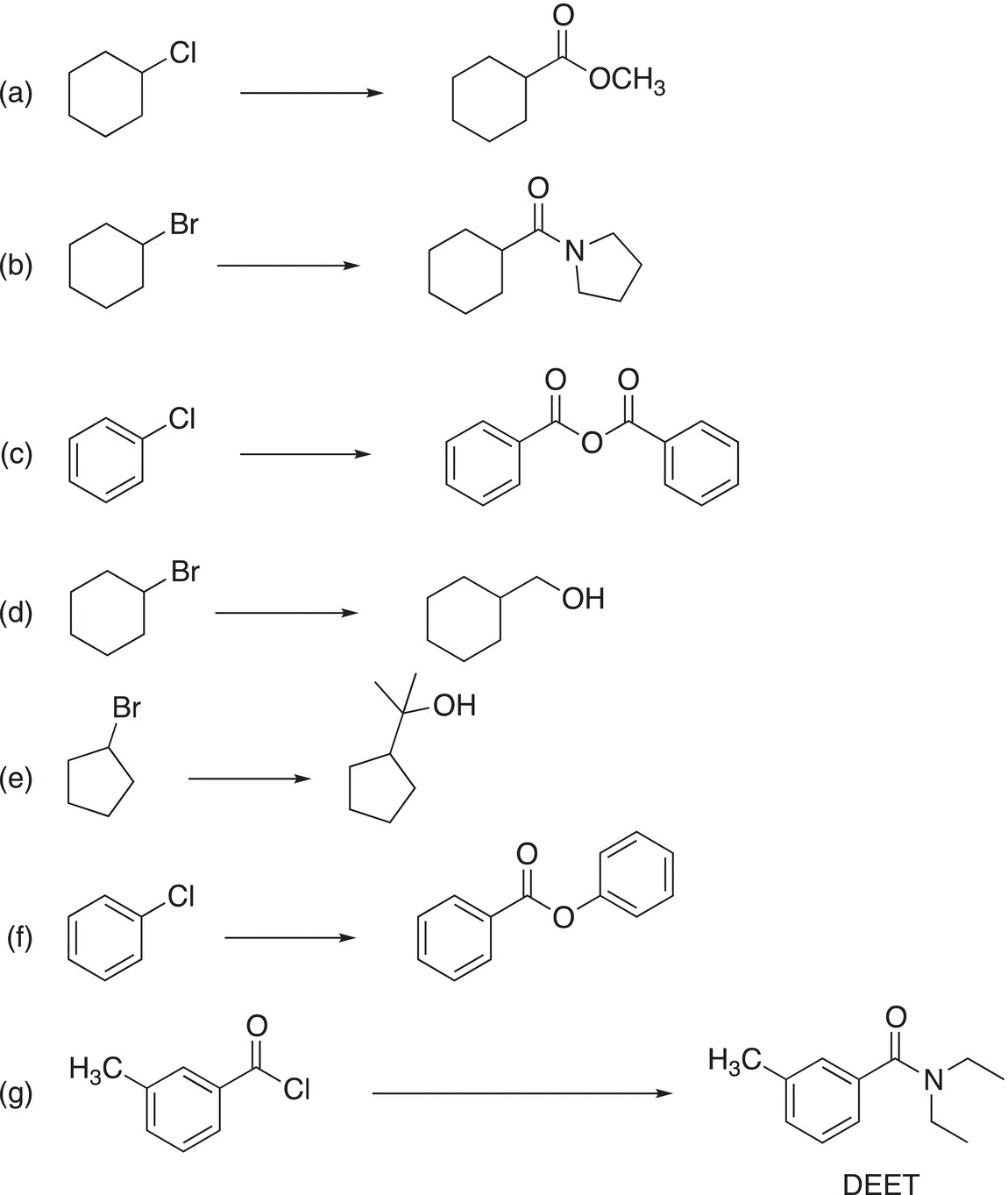
4. 16.34 Use an appropriate mechanism (arrow pushing) to account for the fact that when a carboxylic acid is dissolved in water labeled with 18O, the reaction shown below is observed.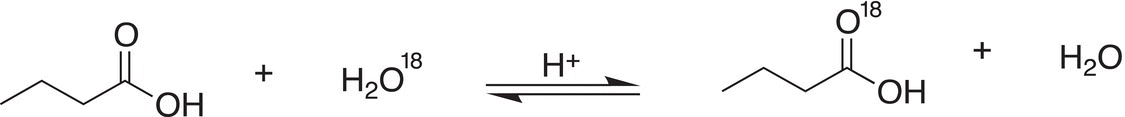
5. 16.35 Give the major organic product, which are all lactones, of the following reactions.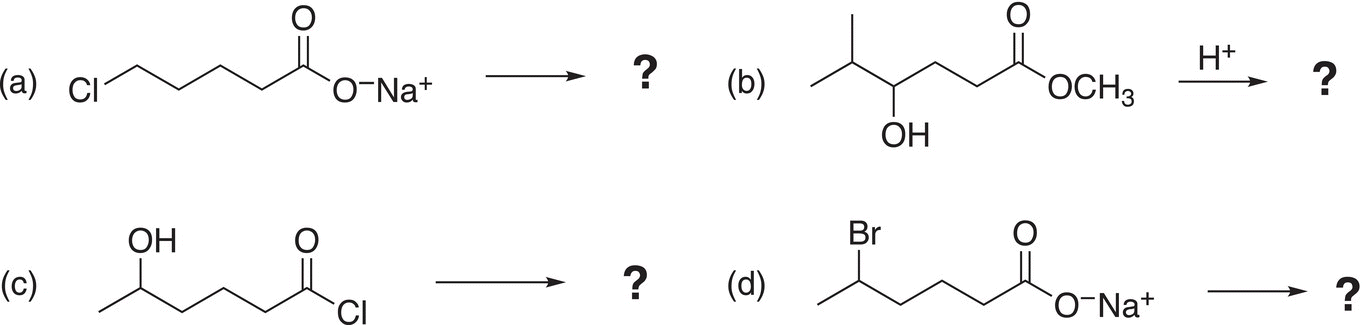
6. 16.36 Determine which of the reactions shown below is more favorable, explain your answer.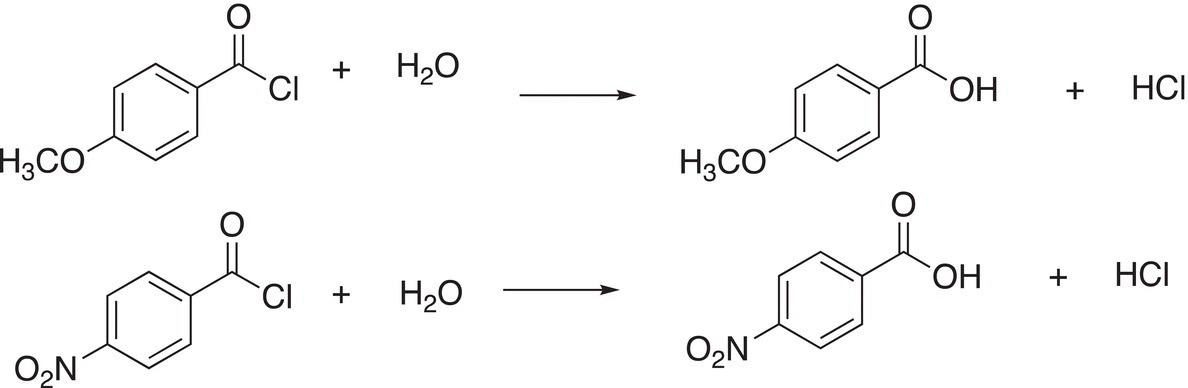
7. 16.37 Show how to carry out the following transformation. N,N-Diethyl meta-toluamide (DEET) is the main ingredient in insect repellent.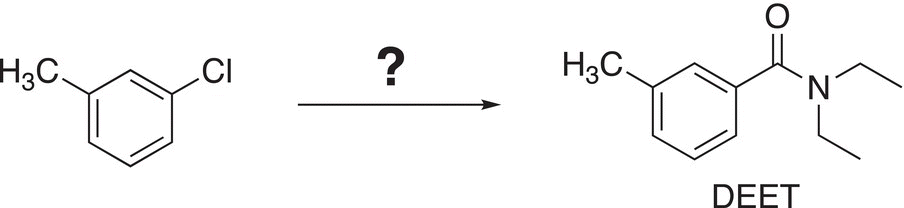
8. 16.38 Complete the following reactions by giving the structures of the missing reactant, reaction conditions, or major organic product. Indicate stereochemistry where appropriate.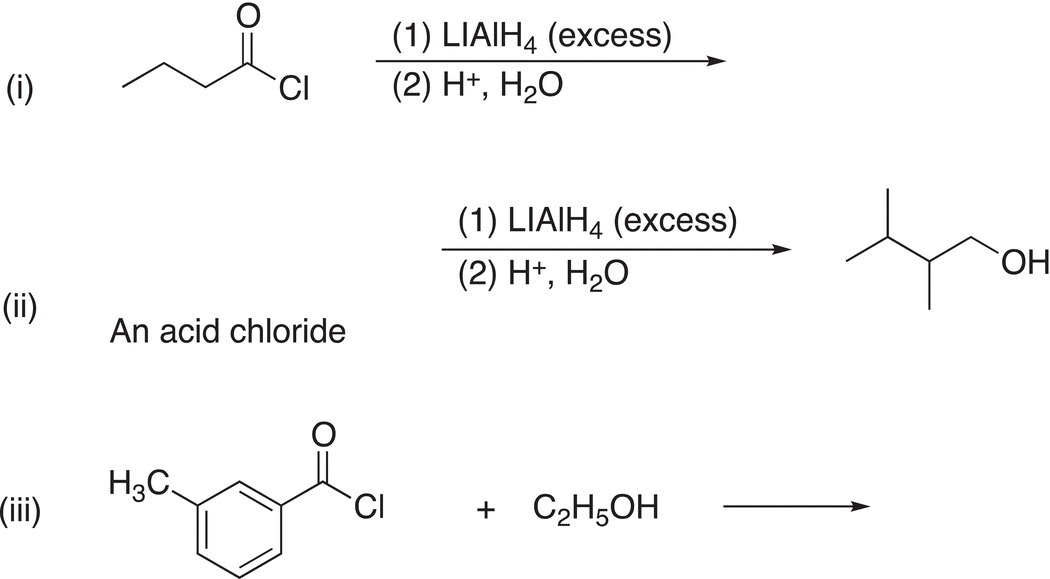
9. 16.39 Sulfamethoxazole is an antibacterial compound from a group of compounds called sulfa drugs, which are widely used as antibacterial agents, its structure is shown below. Determine the structure of the amine necessary to carry out the transformation for its synthesis. Also determine how to remove the protecting group for the amine functionality that is bonded to the phenyl ring to give the target sulfamethoxazole molecule.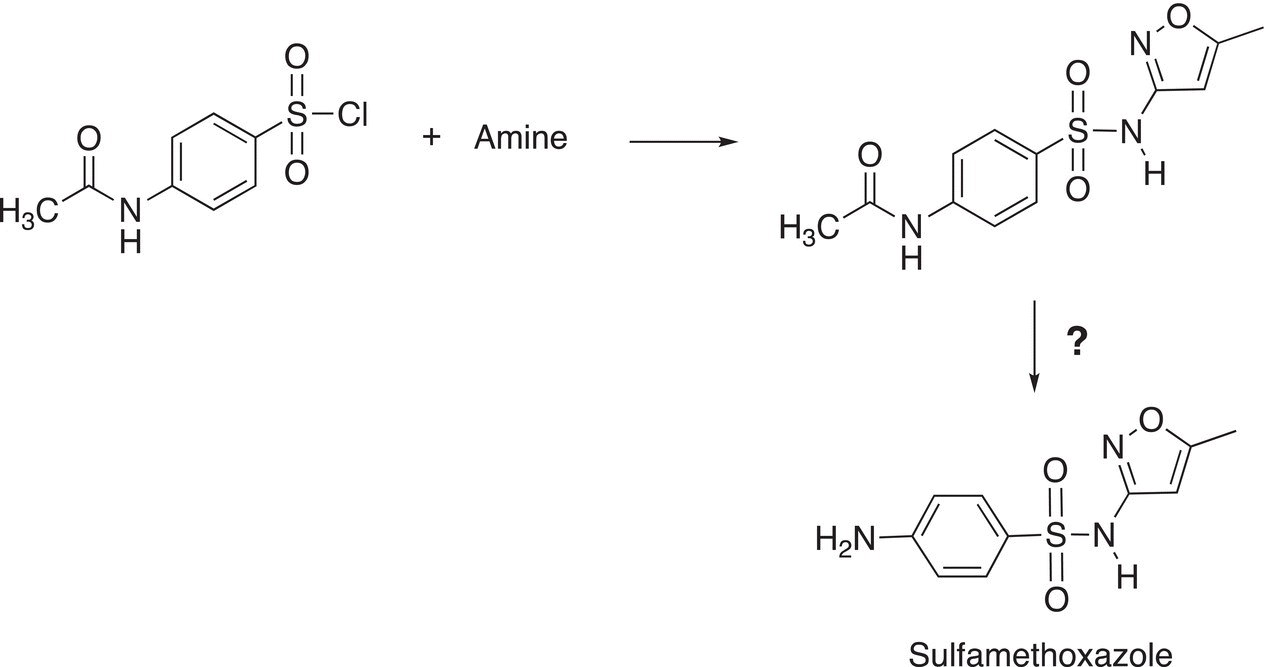
10. 16.40 Using arrow formulism to indicate electron movement, provide a step-by-step mechanism for the reaction shown below.