Organic Chemistry: Concepts and Applications - Headley Allan D. 2020
Aromaticity and Aromatic Substitution Reactions
End of Chapter Problems
1. 17.17 Which of the following molecules is(are) aromatic? Explain your answer.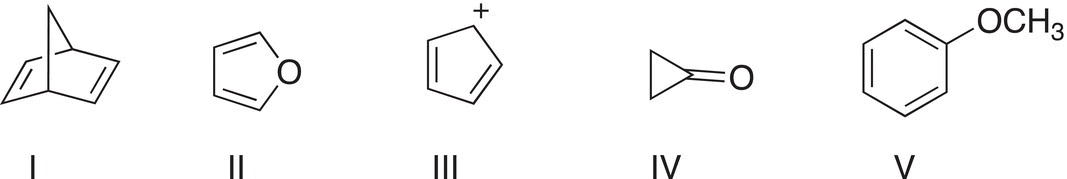
2. 17.18 Give IUPAC names for the following compounds.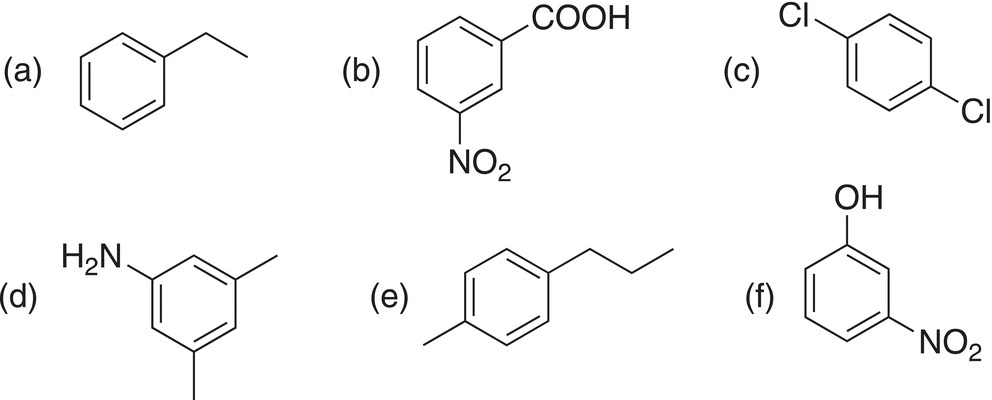
3. 17.19 Give the structure of each of the compounds shown below.
a) 1-Bromo-2-chlorobenzene |
e) 2,4,6-Trinitrotoluene |
b) 1-Iodo-2,3-dimethylbenzene |
f) p-Chlorobenzoic acid |
c) m-Chlorophenol |
g) o-Nitrophenol |
d) 2,3-Dichlorophenol |
h) 4-Chloroaniline |
4. 17.20 Give the structure of the expected principal organic product when benzene reacts with the specified set of reagents.
a) HNO3/H2SO4 |
c) Cl2/FeCl3 |
b) CH3COCl/AlCl3 |
d) SO3/H2SO4 |
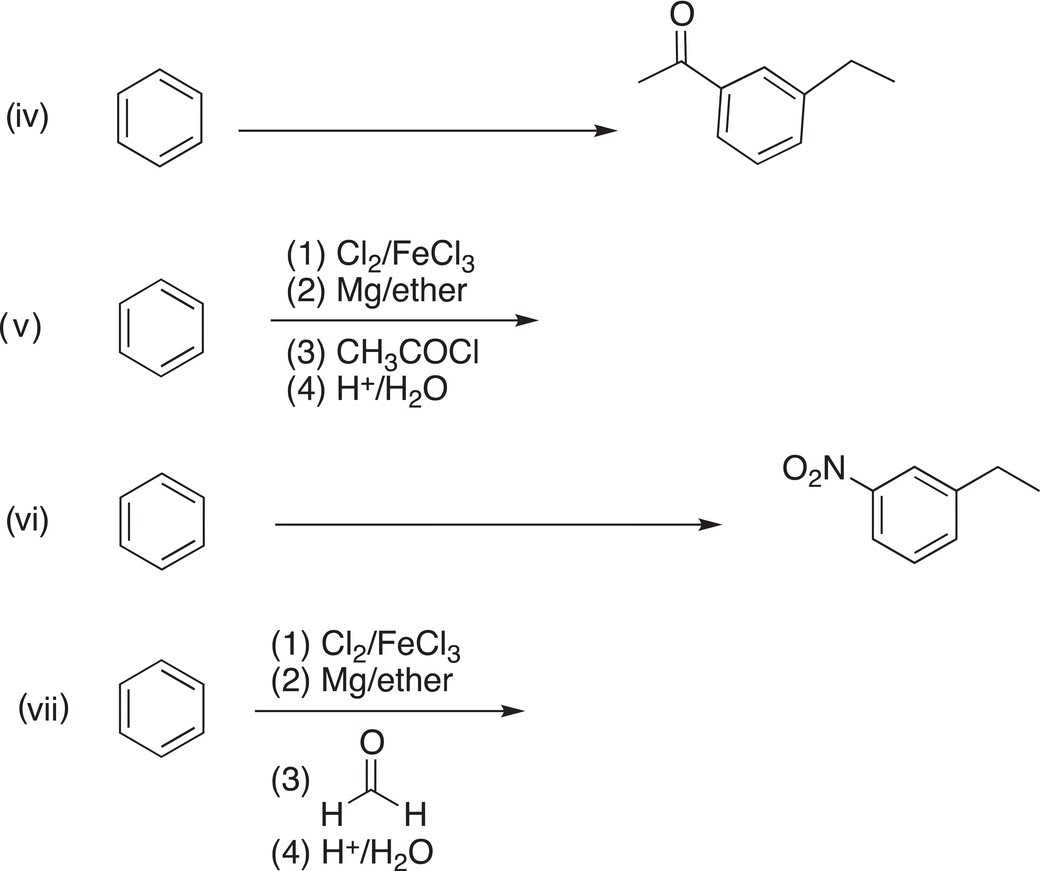
5. 17.21 Complete the following reactions by giving the structures of missing reagents above the arrow or major organic product. Note: some transformations may require more than one step and these steps must be indicated clearly.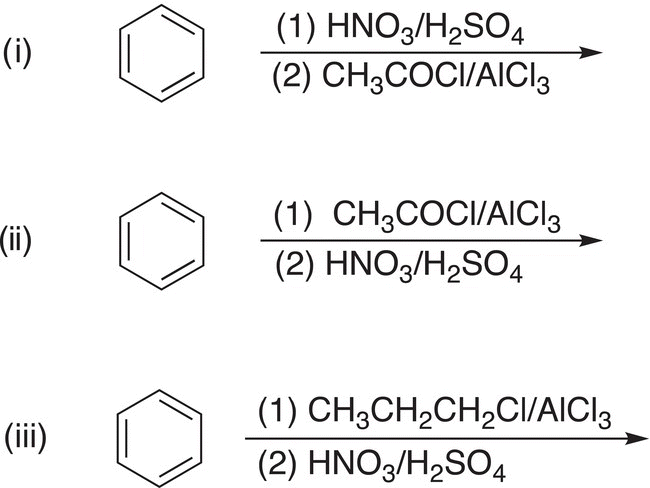
6. 17.22 Using arrow-pushing formulism to indicate electron movement, propose a reasonable mechanism to account for the following observation.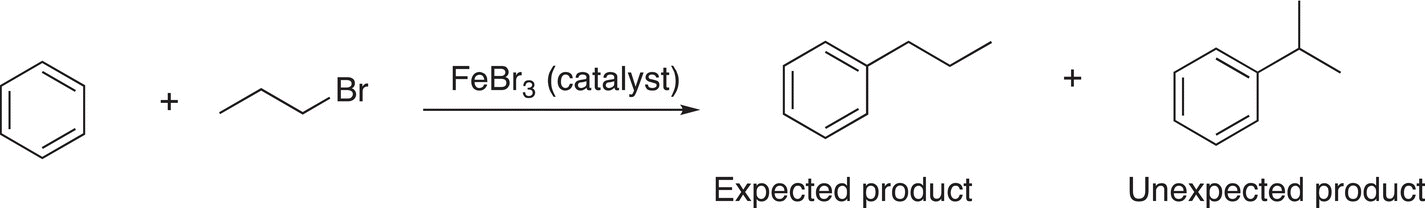
7. 17.23 Provide the necessary reagents and reaction conditions needed to carry out each lettered transformation (note: it may be necessary to have more than one step to accomplish a particular transformation). For example: (i) C6H5MgCl/ether, (ii) H3O+.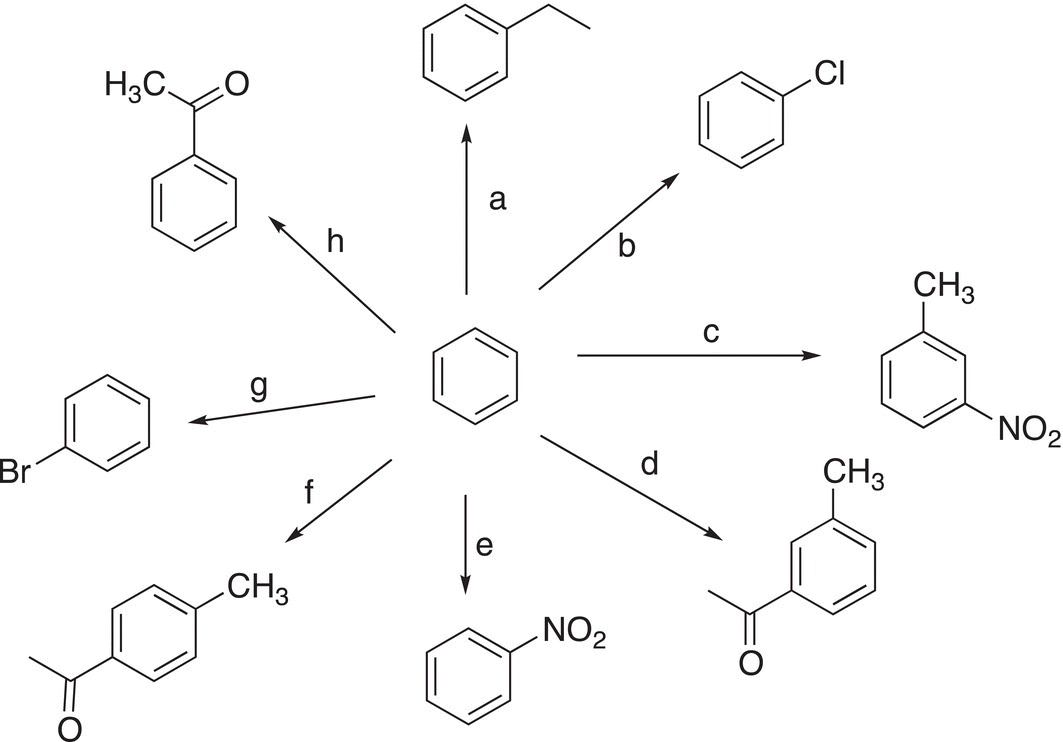
8. 17.24 Using arrow-pushing formulism to indicate electron movement, provide a step-by-step mechanism for the following reaction.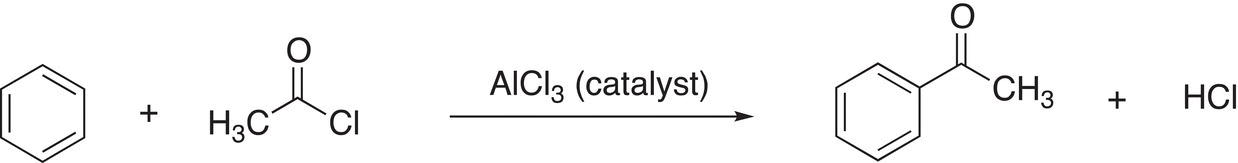
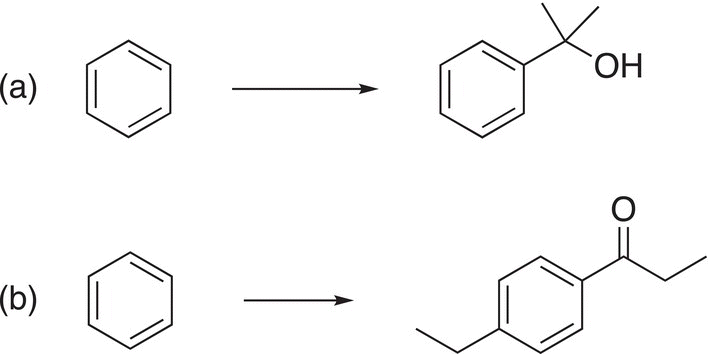
9. 17.25 Indicate clearly, by giving appropriate reaction conditions, how you would carry out the transformations shown below. Note that some transformations may require more than one step.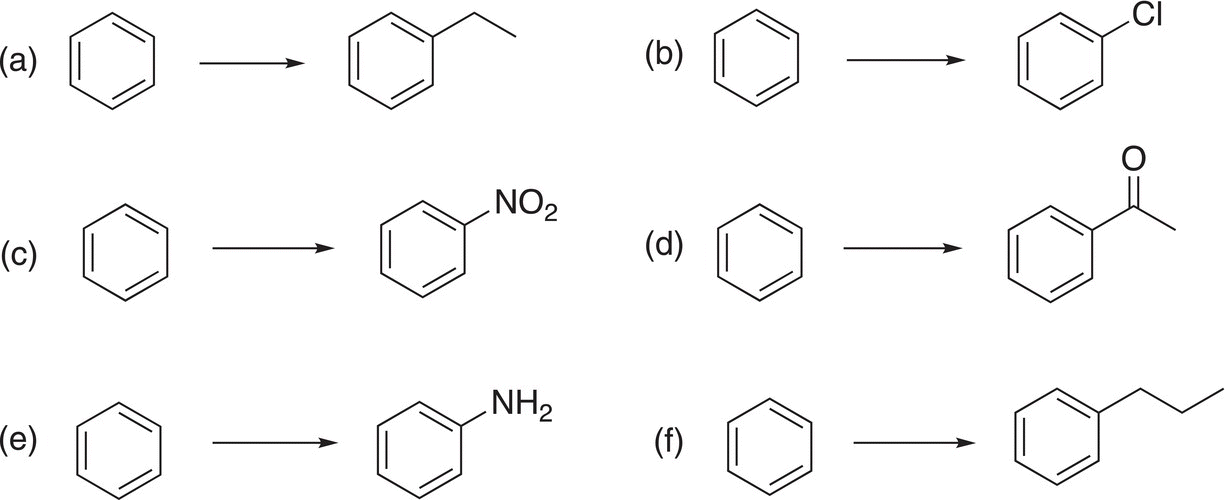
10. 17.26 Provide the reagents and reaction conditions necessary to carry out the transformations shown below.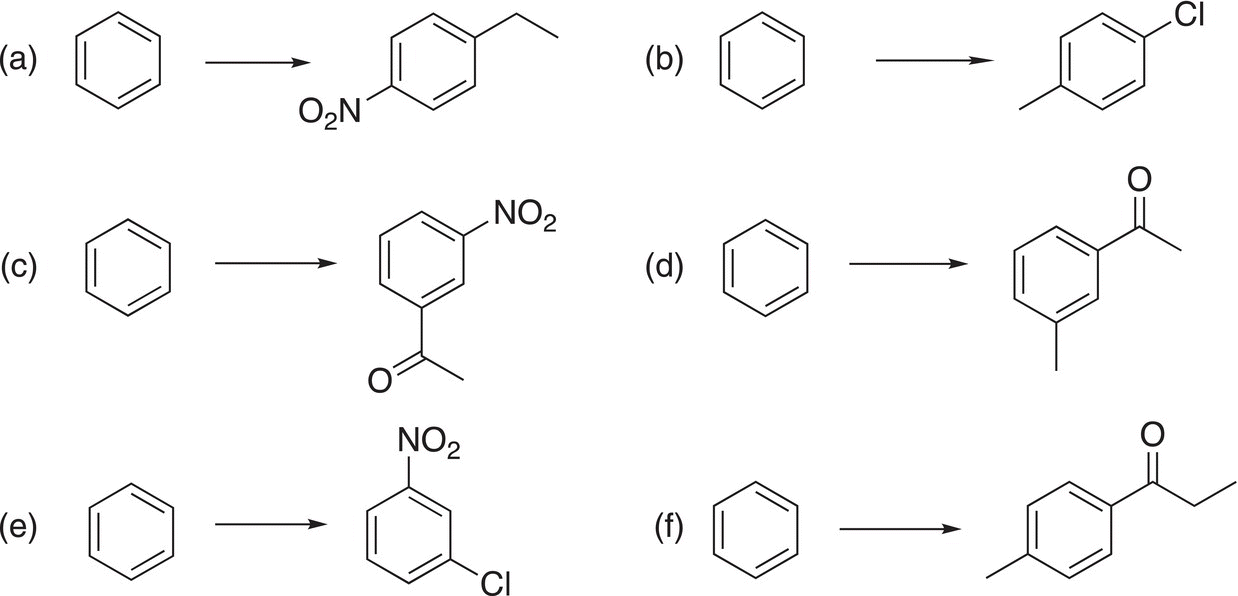
11. 17.27 Give the structures of the expected principal organic products when naphthalene reacts with the specified set of reagents.
a) H2SO4/HNO3 |
c) Cl2/FeCl3 |
b) CH3COCl/AlCl3 |
d) SO3/H2SO4, 60 oC |
12. 17.28 Give the structure of the expected principal organic product when pyrrole reacts with the specified set of reagents.
a) H2SO4/HNO3 |
c) Cl2/FeCl3 |
b) CH3COCl/AlCl3 |
d) SO3/H2SO4 |
13. 17.29 Picric acid is one of the strongest organic acids, with a pKa = 0.3. Draw resonance structures for the molecule shown below and explain its acidity.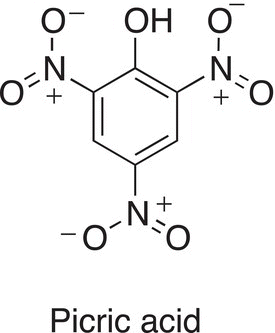
14. 17.30 Using appropriate mechanisms, account for the following observations.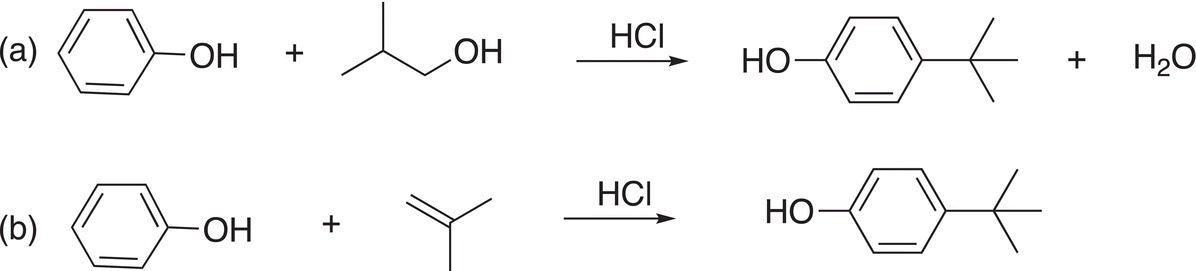
15. 17.31 Explain the following observation.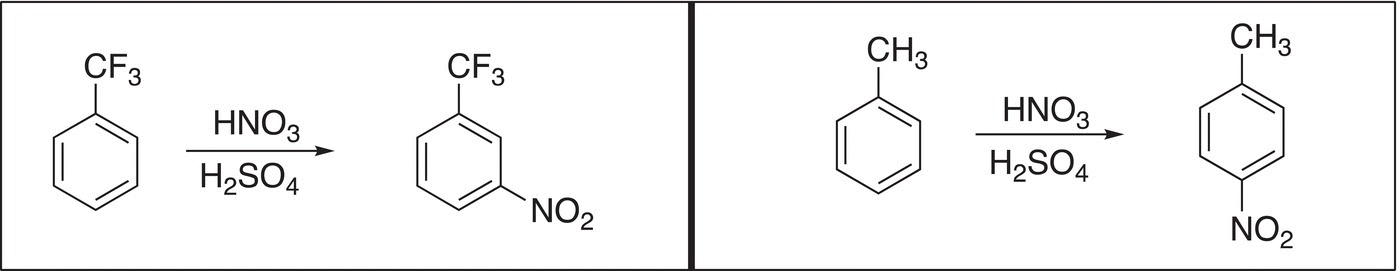
16. 17.32 Give a mechanism to explain the reaction shown below.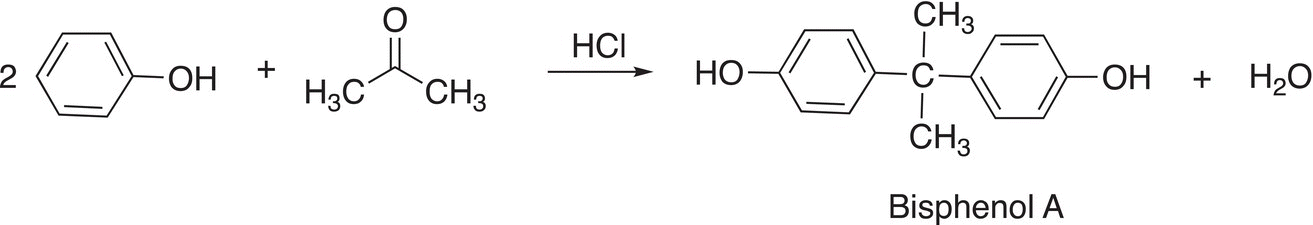
17. 17.33 Give the missing reagents to complete the synthesis of benzocaine hydrochloride, a reagent that is routinely used as an anesthetic.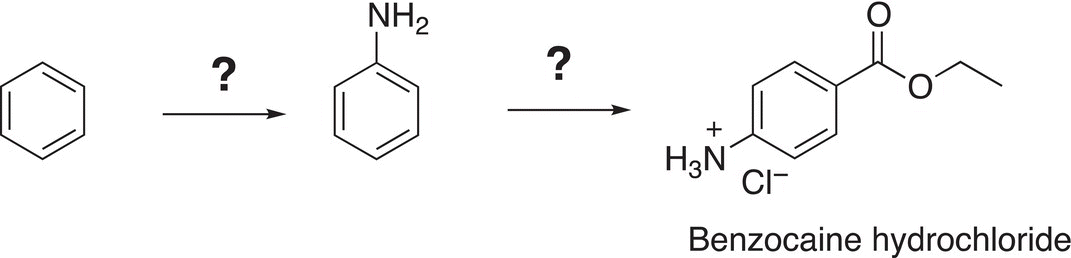
18. 17.34 Provide a mechanism to explain the following reaction, this mechanism is similar to the mechanism for the Friedel—Crafts acylation. (hint: in the first step, consider using a lone pair of electrons on the carbonyl oxygen to bond to the vacant orbital of the Lewis acid catalyst, AlCl3).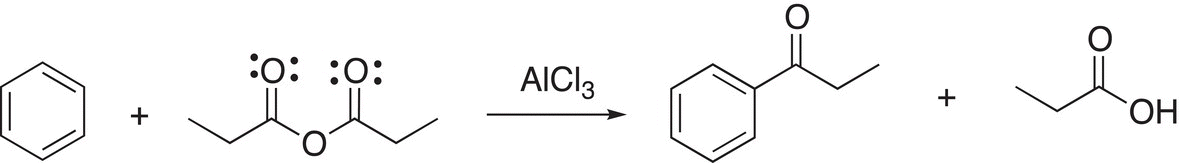
19. 17.35 A possible synthesis for ibuprofen is given in Reactions (17-54) to (17-56). An organic chemistry student proposed that a slight modification to ibuprofen should still make it to be effective and the structure is shown below. Using benzene as your starting compound, show how to synthesize this modified ibuprofen.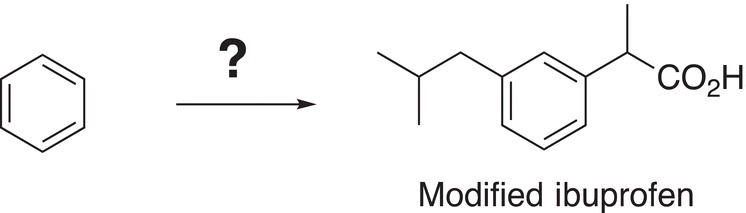
20. 17.36 It was mentioned that the SO3H group can be used as a protecting group for a specific position on the benzene ring. Provide reagents and appropriate reaction conditions to carry out each of the lettered transformation.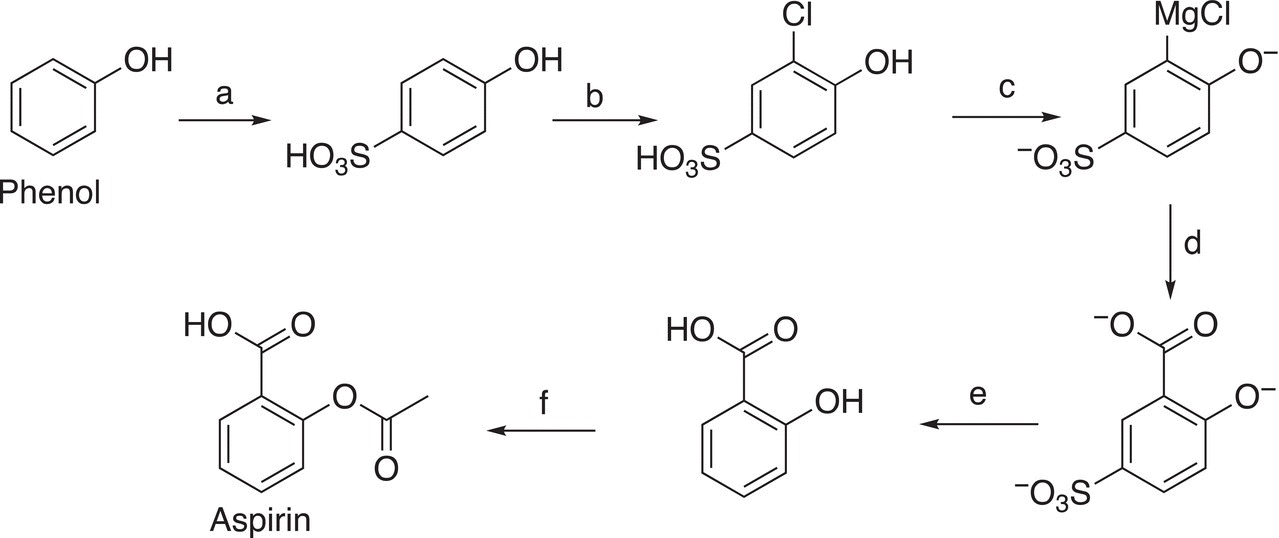
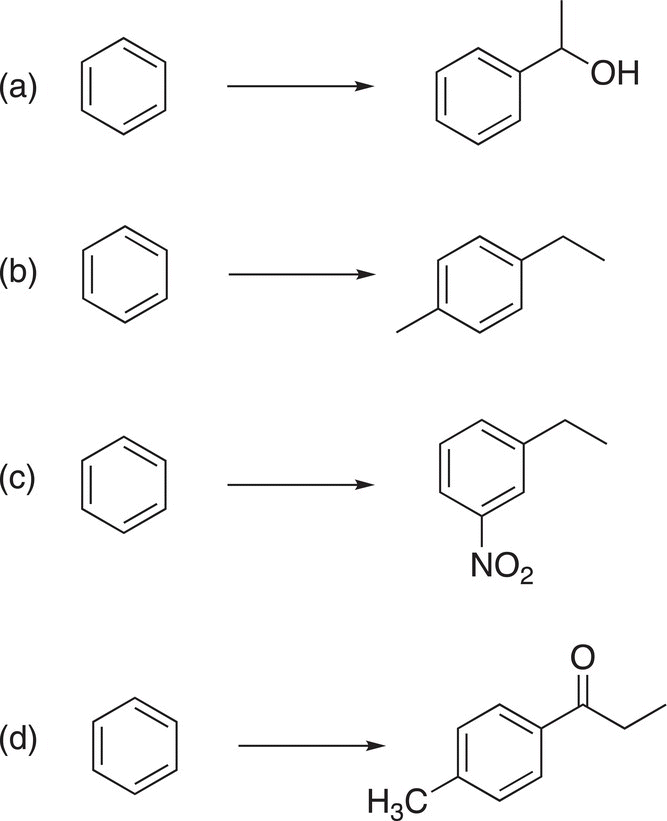
21. 17.37 Show how to carry out the following transformations. For each step in your synthesis, clearly show all reagents and reaction conditions. (hint: identify the bond of the functional group in the product that must be made, then work backward to identify an appropriate molecule and reaction type to make that bond).
22. 17.38 At temperatures in excess of 160 °C, 2,4,5-trichlorophenol, which is a constituent of the warfare defoliant “Agent Orange,” dimerizes to give dioxin. Propose a reasonable mechanism for the reaction shown below.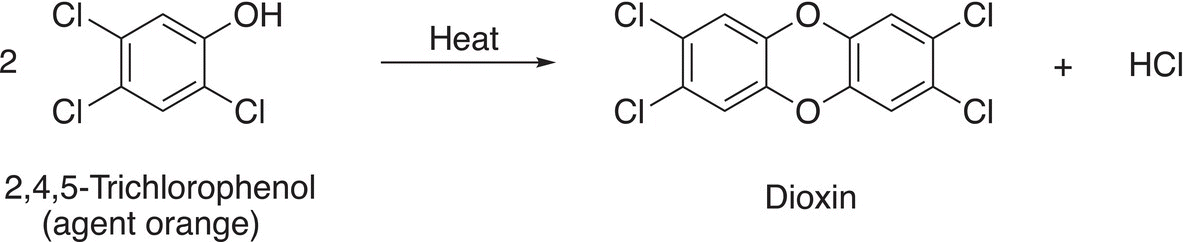
23. 17.39 Determine which of the reactions shown below is most favorable.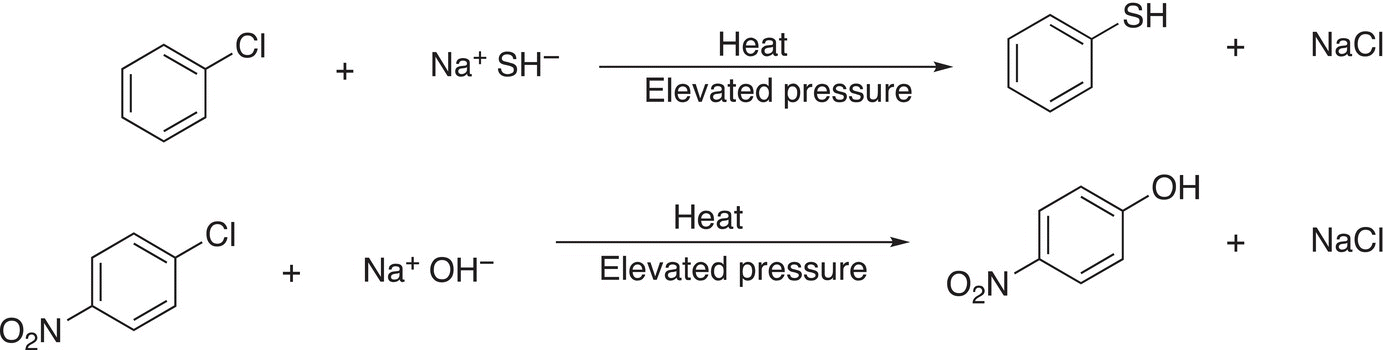
24. 17.40 The 1H NMR spectrum for cyclopentadiene salt consists of just one signal, whereas the NMR spectrum of cyclopentadiene is more complex. Explain this observation.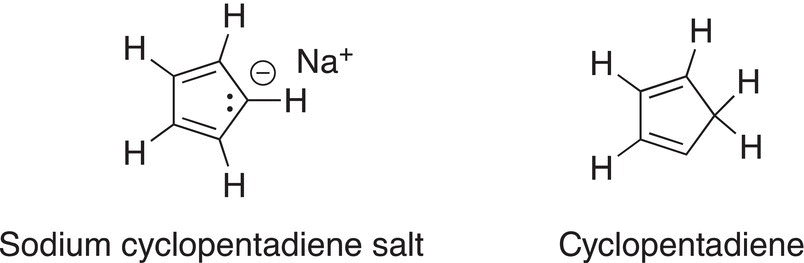
25. 17.41 Show how to carry out the following transformations. For each step in your synthesis, clearly show all reagents and reaction conditions. (hint: identify the bond of the functional group in the product that must be made, then work backward to identify an appropriate molecule and reaction conditions to make that bond).