Organic Chemistry: Concepts and Applications - Headley Allan D. 2020
Conjugated Systems and Pericyclic Reactions
18.1 Conjugated Systems
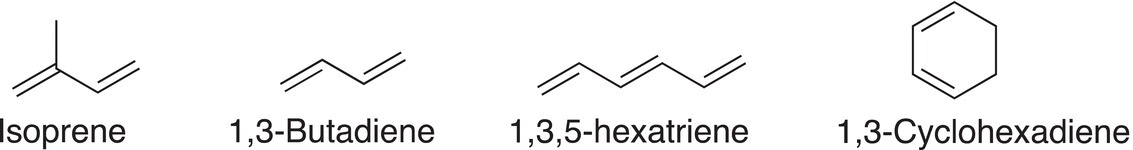
Conjugated systems are found routinely in nature, most are typically highly colored compounds. 2-Methyl-1,3-butadiene, also known as isoprene, is one of the simplest conjugated systems and its structure is shown below. Isoprene is a colorless liquid that is produced and emitted by many types of trees, including the oak and eucalyptus trees. Shown also below are two other examples of simple conjugate compounds.
As we have seen on the section on UV—Vis spectroscopy in Chapter 13, a large percentage of organic compounds, especially natural products, contain extended conjugated double bonds. β-Carotene and lycopene, for example, are highly colored compounds due to the presence of extended conjugated double bonds. In this chapter, we will examine the reactions that conjugated systems undergo and apply that knowledge to the synthesis of various other more complex molecules.
18.1.1 Stability of Conjugated Alkenes
In Chapter 4, we briefly looked at the stability of alkenes based on the heat liberated from hydrogenation reactions. We examined the relationship between the amount of heat liberated to the relative stability of different alkenes. Let us revisit the hydrogenation of different isomers of pentene. Consider the hydrogenation of 1-pentene and 2-pentene, which are shown in Reactions (18-1) and (18-2).
(18-1)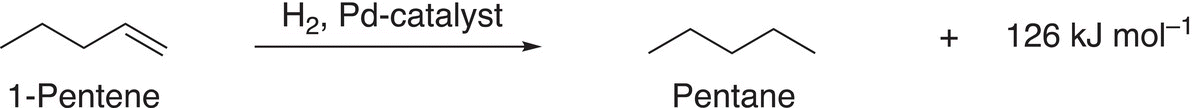
(18-2)
The heat liberated from the hydrogenation of 1-pentene, which has a terminal double bond is 126 kJ mol−1 (Reaction (18-1), is more than the heat liberated from the hydrogenation of 2-pentene, which has an internal double bond. An extrapolation of this information to conjugated systems would imply that the heat liberated from 1,3-pentadiene should be 126 + 114 kJ mol−1 (240 kJ mol−1), which would be the sum of the two reactions shown in Reactions (18-1) and (18-2). Reaction (18-3) shows that the heat liberated is less than predicted, actually 225 kJ mol−1.
(18-3)
The implication is that the conjugated diene is more stable than a molecule that has two isolated double bonds. Another analysis that demonstrates that observation is that the heat liberated from the hydrogenation of 1,4-pentadiene, which has two isolated terminal double bonds, is 252 kJ mol−1, which is the sum of two moles of 1-pentene shown in Reaction (18-1) (126 + 126 kJ mol−1).
(18-4)
The implication from these observations is that molecules that have conjugated double bonds are more stable than molecules that have isolated double bonds. In the case of pentadienes, 1,3 pentadiene is more stable than 1,4-pentadiene, which has isolated double bonds. Figure 18.1 gives the energy diagram for this concept.
The stability of the conjugated alkene comes from the fact that the electrons of the double bond can freely delocalize across the pi (π) system. We can use resonance to illustrate this concept as shown in resonance structures given in (18-5) and (18-6).
(18-5)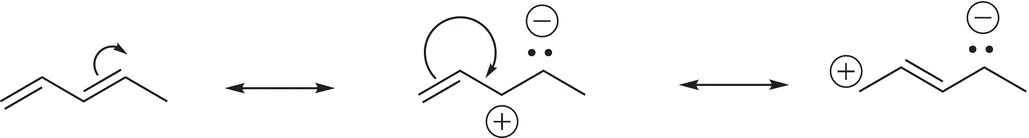
(18-6)
An alternate representation of the electronic distribution is shown below.
 ΔΔ
ΔΔ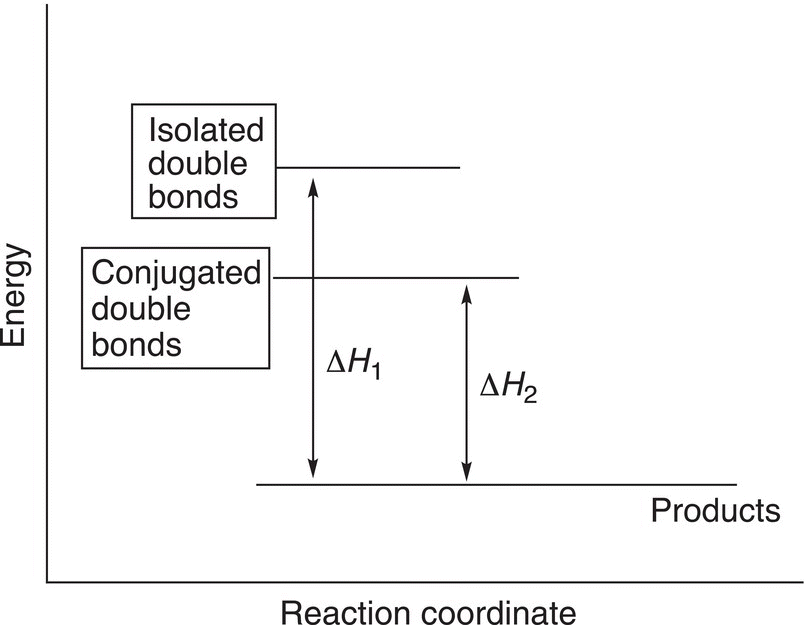
Figure 18.1 Energy diagram of the relative stability of molecules with conjugated double bonds compared to molecules with isolated double bonds.
As we have seen from various addition reactions studied thus far, unexpected products are sometimes produced owing to the possibility of resonance, an example is shown in Reaction (18-7).
(18-7)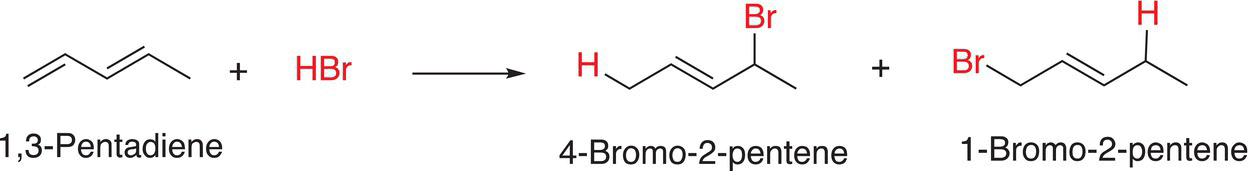
The same type of electron delocalization is true for molecules that have atoms with unshared electrons adjacent to a double bond, as shown in the resonance structures given in (18-8).
(18-8)
Problem 18.1
i. Determine which of the following molecules or ions have conjugated electrons?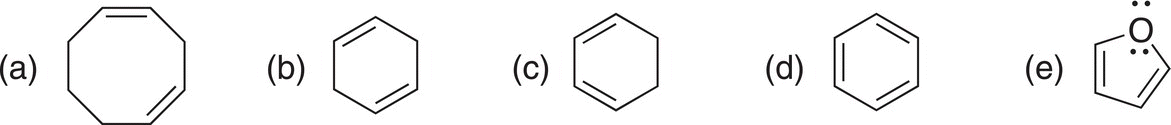
ii. For structures that are conjugated, use arrows to show electron movement that results in another resonance structure.