Organic Chemistry: Concepts and Applications - Headley Allan D. 2020
Catalytic Carbon—Carbon Coupling Reactions
19.2 Reactions of Transition Metal Complexes
In this section, we will examine the structure and type of reactions that selected coordination transition—metal complexes undergo. Some of these reactions are already mentioned in previous chapters, but in this section, more details will be provided about the mechanism for these reactions.
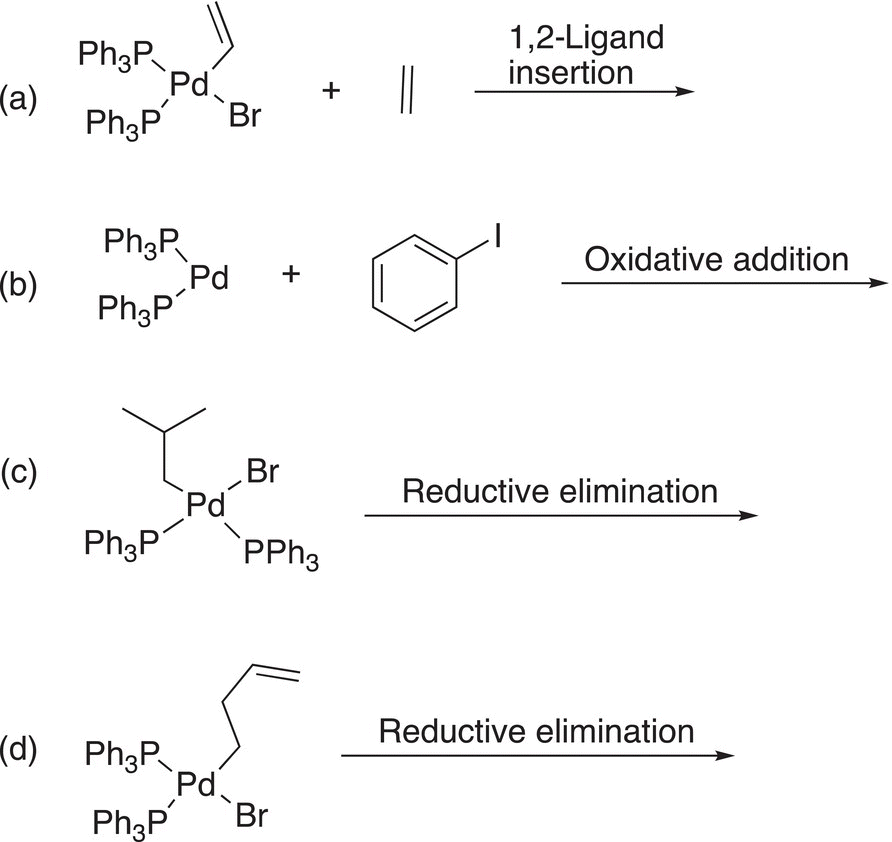
19.2.1 Oxidative Addition Reactions
In oxidative addition, a metal is inserted into a sigma bond. We have already seen this type reaction in the synthesis of the Grignard reagent, in which magnesium is inserted into the C─X bond to form R-Mg-X, where X is a halogen. For Reaction (19-1), the metal Mg in the reactant is oxidized to Mg2+. As a result, the term oxidative addition is used to describe this type of reaction.
(19-1)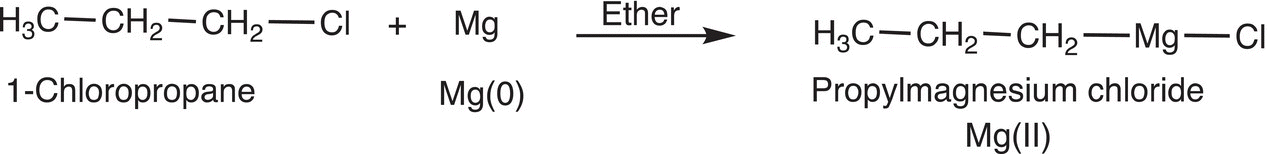
As we have mentioned in earlier chapters, Victor Grignard discovered this type of reaction, and the reaction is now known as the Grignard reaction and the organomagnesium halides generated are known as Grignard reagents.
For transition metals, the same type of reaction is possible as shown below in Reaction (19-2), in which Pd is inserted into a Ph─X bond, where Ph is the phenyl group (—C6H5) and X is a halogen.
(19-2)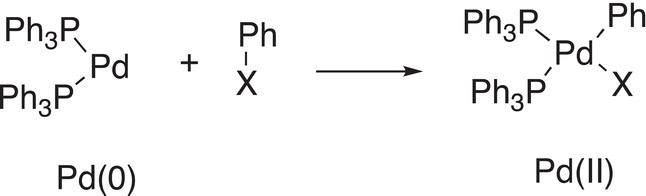
Note the change in oxidation state of palladium, it is Pd(0) in the reactant and is oxidized to Pd(II) in the product. The formation of this type of complex is key to the reactions that will be discussed throughout this chapter.
19.2.2 Transmetallation Reactions
Another important reaction that coordinated transition—metal complexes can undergo is transmetallation as shown in Reactions (19-3) through (19-5a).
(19-3)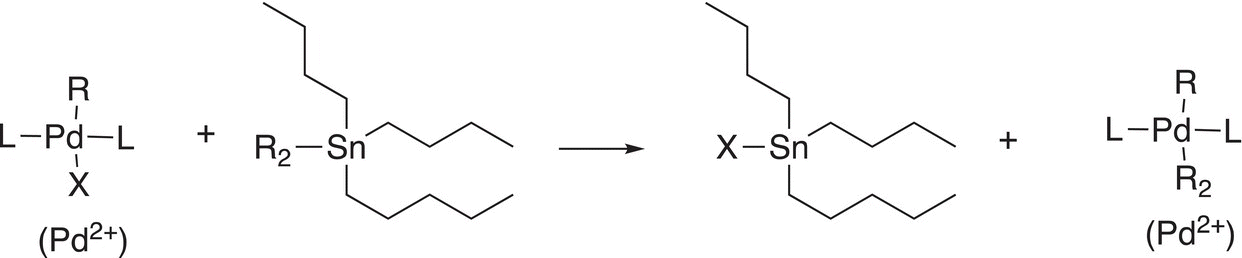
(19-4)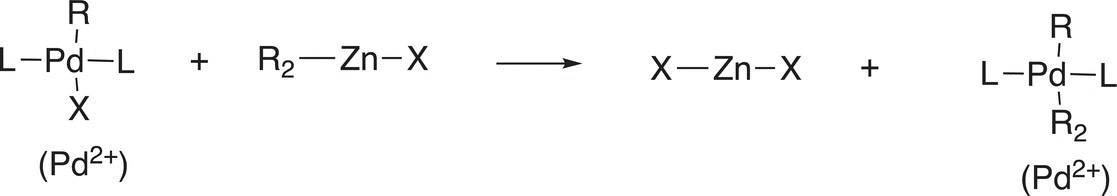
(19-5a)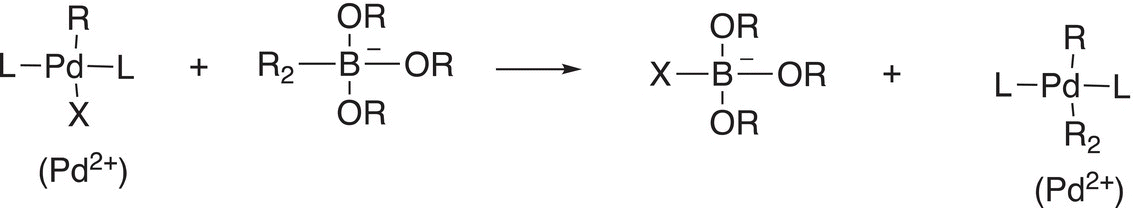
These reactions are very straightforward in that one group is transferred from a coordinated metal ion complex to another. We have seen a similar type of reaction before in the synthesis of organocuprates, also known as the Gillman reagent and given in Reaction (19-5b).
(19-5b)
19.2.3 Ligand Migratory Insertion Reactions
Another type of a reaction of metal ion complex is the possibility of migratory insertion of a ligand in a bond of the metal ion, as shown below in Reaction (19-6).
(19-6)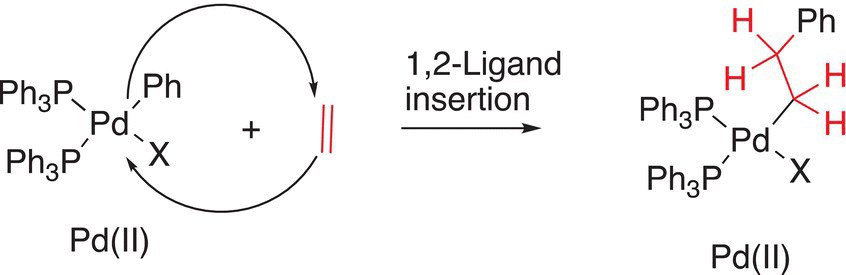
These reactions are called 1,2 insertion since the insertion takes place across two carbons of the alkene, and this type of reaction is also key in coupling reactions, as we will see later in the chapter.
19.2.4 β-Elimination Reactions
As the name implies, these reactions involve the migration of a atom or group in the β-position of the metal along with its bonding electrons, to the metal. This reaction is essentially the reverse of ligand insertion as discussed in the previous section. An example is shown below in Reaction (19-7).
(19-7)β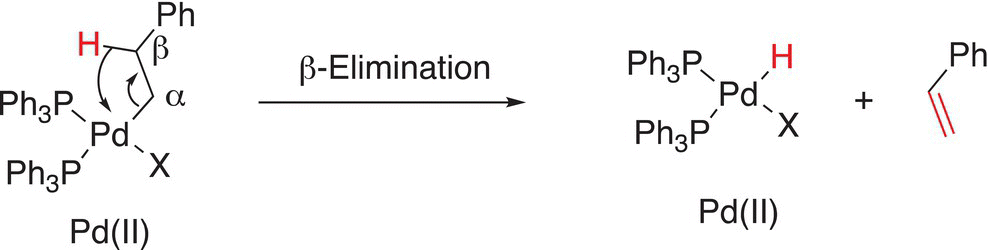
This type of elimination is also known as an intramolecular syn-elimination.
19.2.5 Reductive Elimination Reactions
As the name implies, this type of reaction is essentially the reverse of oxidative addition. For these reactions, two ligands bonded to the metal leave. An example is given in Reaction (19-8).
(19-8)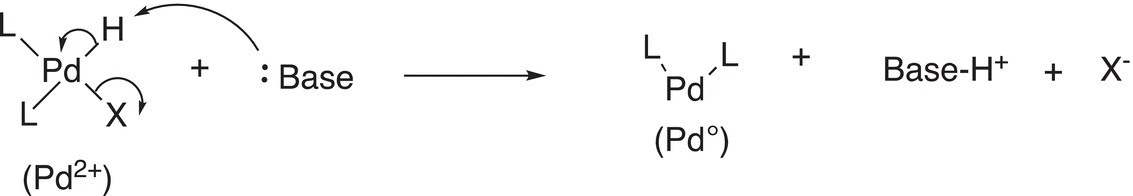
For Reaction (19-8), a base is introduced to abstract a proton and the bonding electrons are used to reduce the Pd2+ to Pd (0) metal while the other ligand leaves with its bonding electrons. Another example of this type of reductive elimination reaction is given in Reaction (19-9).
(19-9)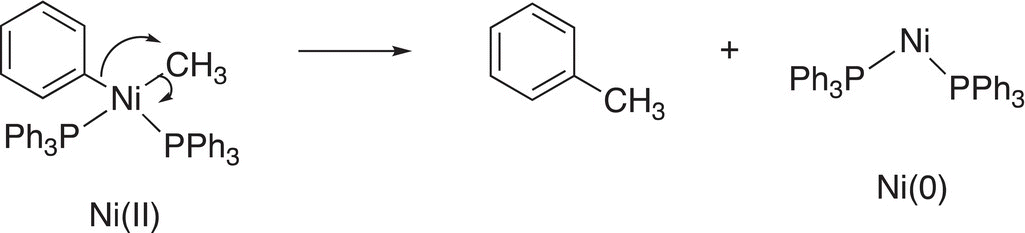
For this reaction, which is an intramolecular elimination, the phenyl group migrates to form a new carbon—carbon bond while the Ni is reduced from Ni(II) to Ni(0).
Problem 19.1
For each of the following reaction described above the arrow, give the products.