Organic Chemistry: Concepts and Applications - Headley Allan D. 2020
Catalytic Carbon—Carbon Coupling Reactions
19.3 Palladium-Catalyzed Coupling Reactions
19.3.1 The Heck Reaction
This first coupling reaction that we will examine in which a transition metal is used is the Heck reaction. This reaction is named after American chemist, Richard Frederick Heck, who is known for the discovery of this reaction. This reaction involves the coupling of aryl halides with alkenes in the presence of a base and a palladium catalyst. A general example of the Heck reaction is shown in Reaction (19-10a).
(19-10a)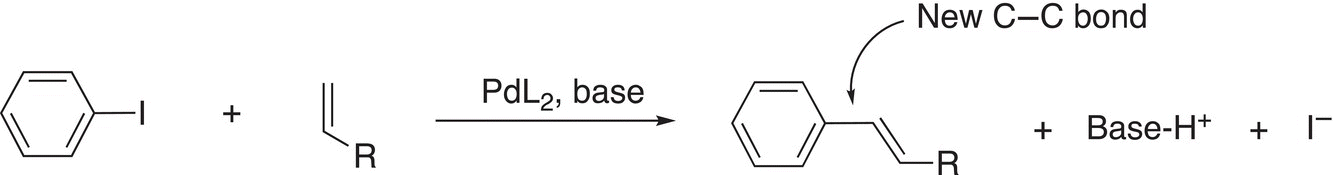
The first step of this reaction involves an oxidative addition of the organoiodide and the Pd complex, as shown in Reaction (19-10b).
(19-10b)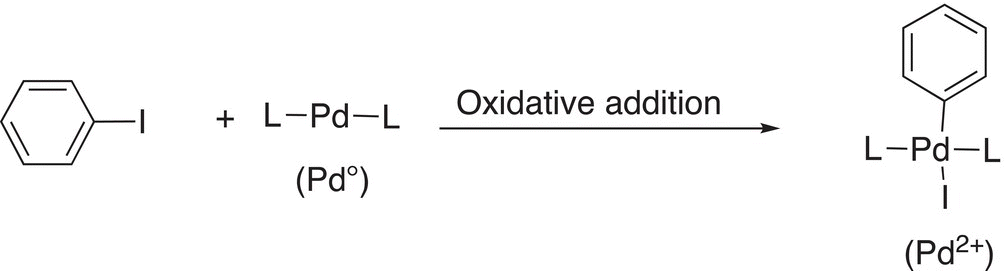
Note that the Pd changes oxidation state from 0 to 2+, hence an oxidation. In the next step of the reaction, the alkene is inserted into the Pd-phenyl bond, as shown in Reaction (19-11).
(19-11)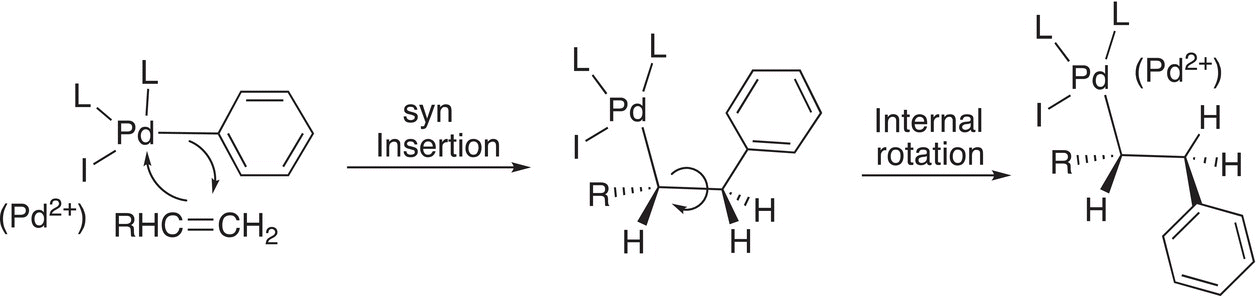
The next step involves a β-hydride elimination, as shown in Reaction (19-12). Note that the elimination is a syn-elimination in that the hydride and Pd must be coplanar in order for the reaction to take place.
(19-12)β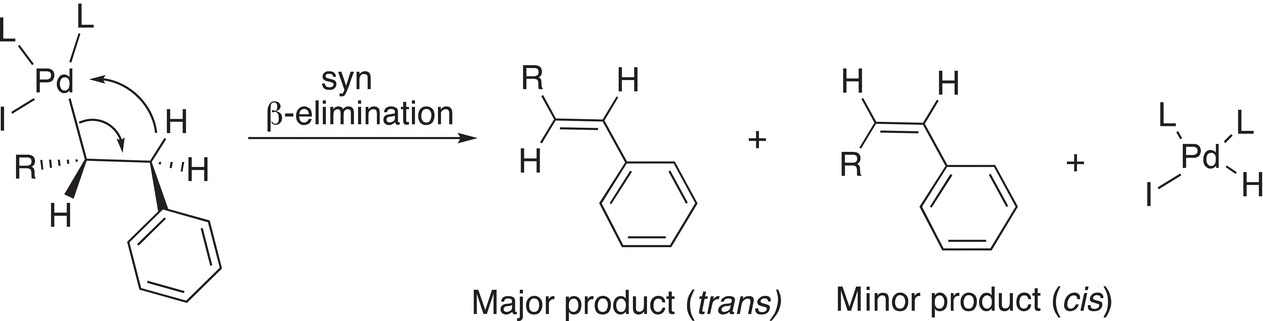
The final step of the reaction is a reductive elimination reaction as shown in Reaction (19-13).
(19-13)
An amine base is typically introduced, which abstracts a proton, and the bonding electrons are used to reduce the Pd (II) to Pd (0), and simultaneously, the iodine being a good leaving group leaves to form the iodide anion. A summary of the reactions for the Heck reaction is given in Figure 19.1.
A specific example of the Heck reaction is shown in Reaction (19-14).
(19-14)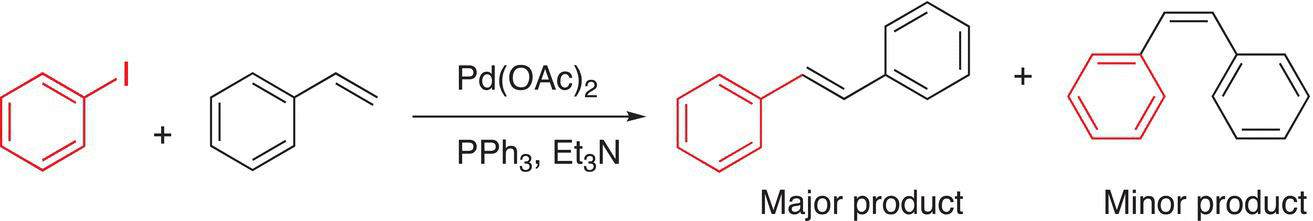
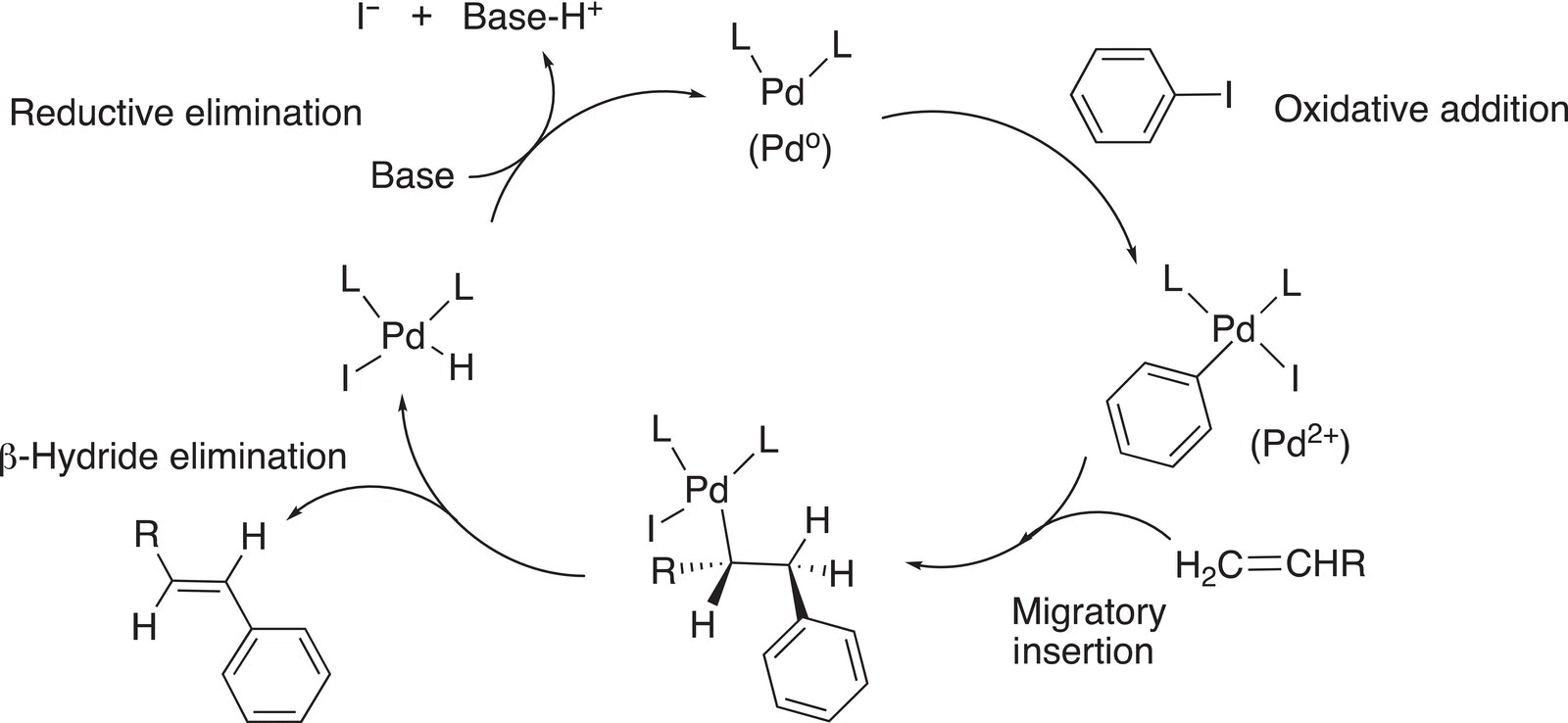
Figure 19.1 A summary of the Heck catalytic coupling reactions.
In this case, the alkene is not symmetrical, and as a result, there are two possible products as shown. The coupling reaction typically occurs at the less substituted end of the alkene and the major product typically has the trans-stereochemistry. The base, triethylamine, is used for the reductive elimination reaction. The Heck reaction is a very important reaction, especially in the pharmaceutical industry; a lot of pharmaceutical products or intermediates leading to the final product have the alkene functionality that can be synthesized using this type of reaction. In addition, since only small amounts of catalysts are needed for these reactions, it can be regenerated at the end of the reaction and recycled.
DID YOU KNOW?
The Heck reaction is used in the industrial production of octyl methoxycinnamate, also known as ethylhexyl methoxycinnamate or octinoxate and marketed as trade names Uvinul MC80 and Eusolex 2292. This compound can absorb ultraviolet (UV) light from sunlight and fluorescent sources and is an ingredient in some lip balms and sunscreens.

Problem 19.2
i. Give the product for the following Heck reactions.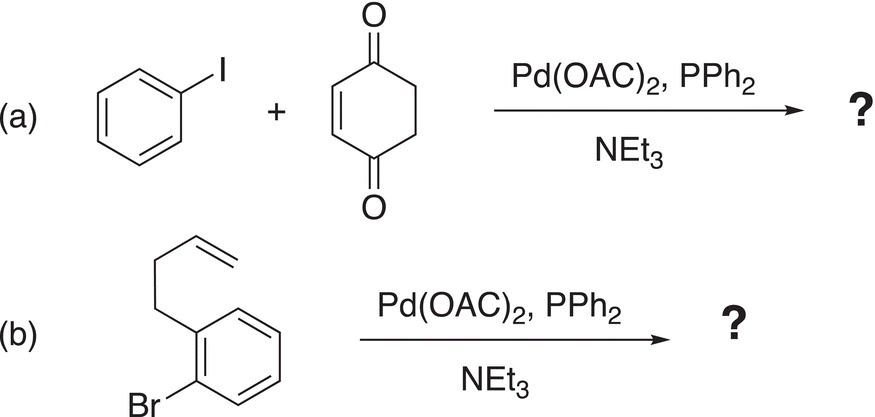
ii. Give the reactants to complete the Heck reactions.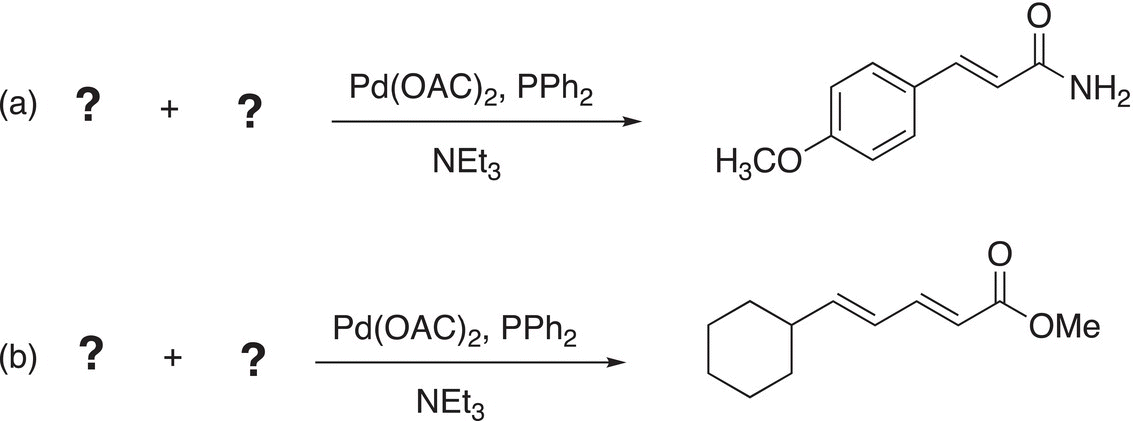
19.3.2 The Suzuki Reaction
The Suzuki reaction is named after Professor Akira Suzuki, a Japanese chemist of Hokkaido University. This reaction is similar to the Heck reaction; but in the Suzuki reaction, organoboranes are used. An example of the Suzuki reaction is given in Reaction (19-15).
(19-15)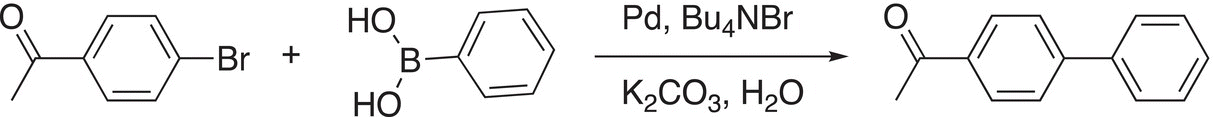
Note carefully for this reaction, like the Heck reaction, a carbon—carbon bond is formed at the point of attachment from the C─Br and C-borane of the reactants. The mechanism to explain this reaction is shown below. The first step of the reaction involves an insertion into the C─Br bond as shown in Reaction (19-6) (oxidative addition).
(19-16)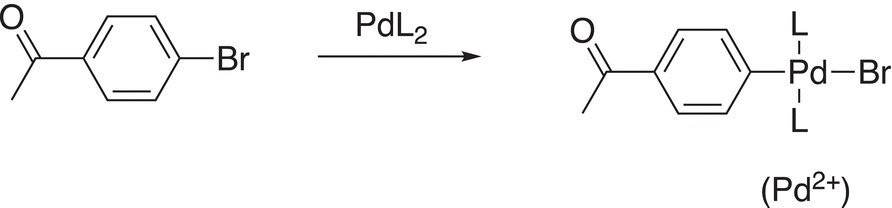
In the next step of the reaction mechanism, boric acid is activated with a base to form a borate complex as shown in Reaction (19-17).
(19-17)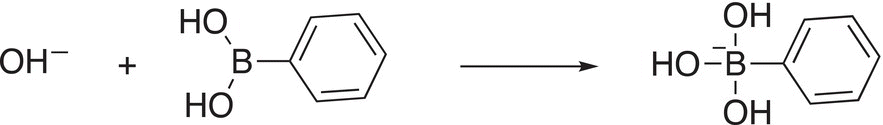
The next step is a transmetallation step, as shown in Reaction (19-18).
(19-18)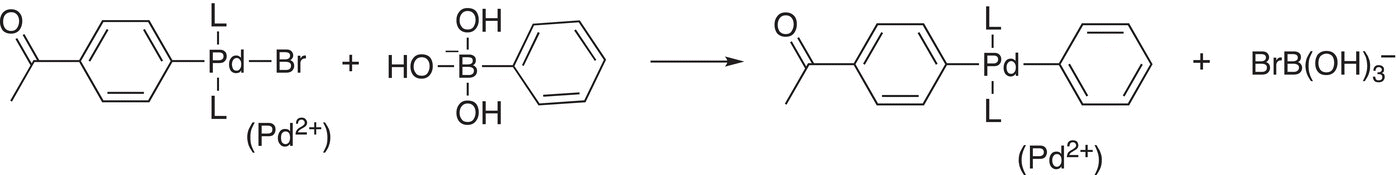
The next step of the mechanism is a reductive elimination in which the catalyst is regenerated as shown in Reaction (19-19).
(19-19)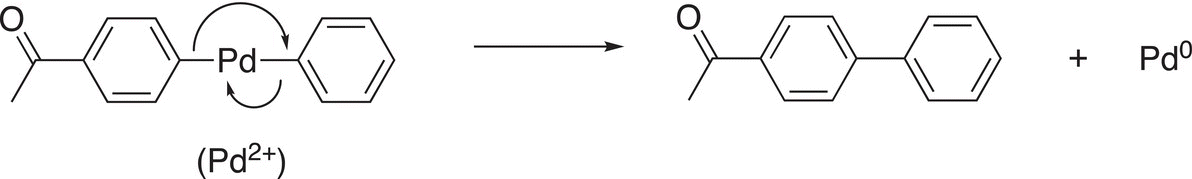
In this step, the catalytic species is regenerated and can be used again. A major advantage of these reactions is that they can be carried out in aqueous media. This is a plus for “Green Chemistry” owing to the advantages of water as a solvent, compared to organic solvents, which are typically toxic and present a disposal problem. Another example of the Suzuki reaction is shown in Reaction (19-20).
(19-20)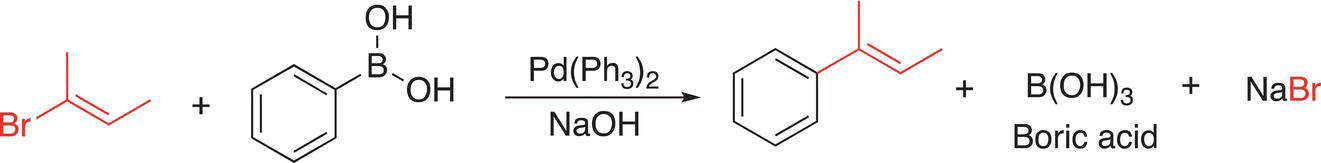
Boronate esters can also be used for the Suzuki reaction, and they can be synthesized as outlined in the example shown in Reaction (19-21).
(19-21)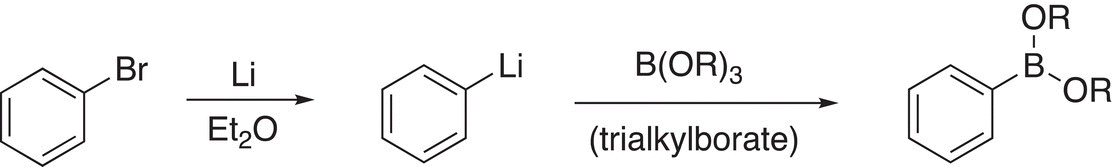
A summary of the Suzuki reaction is shown in Figure 19.2.
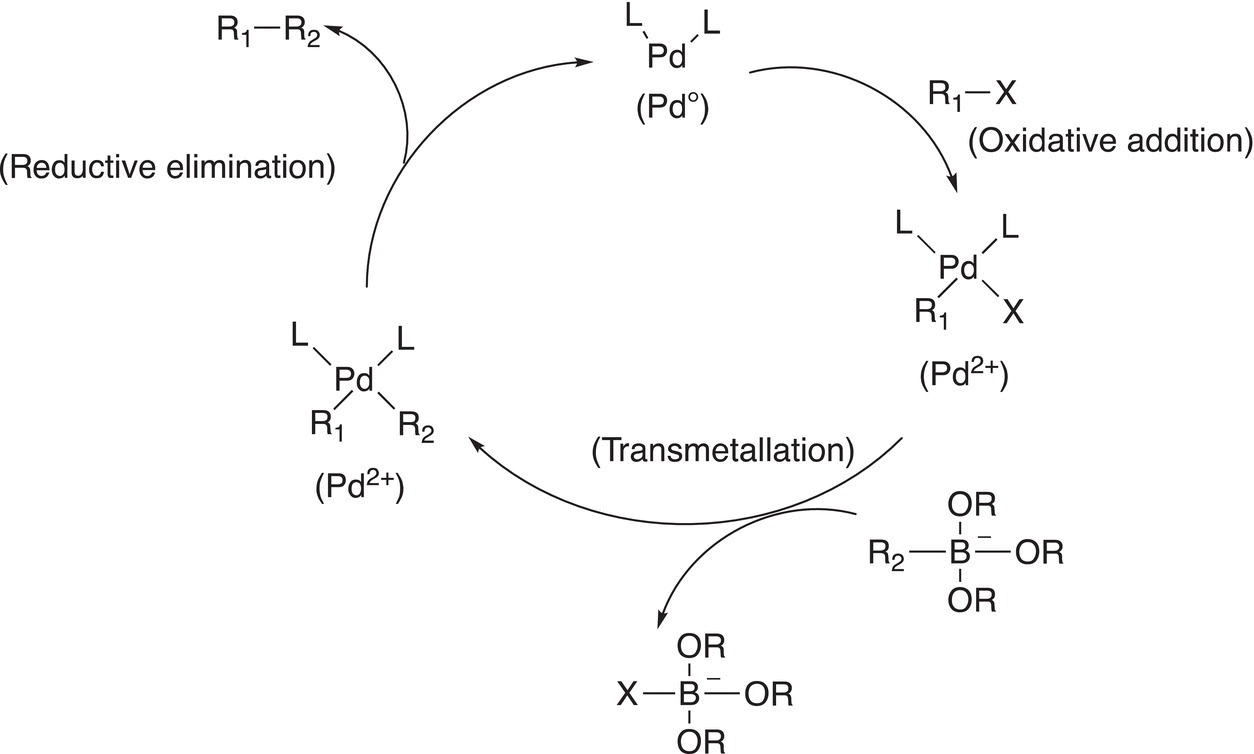
Figure 19.2 A summary of the Suzuki catalytic coupling reactions.
Problem 19.3
i. Give the major organic products to complete the Suzuki reactions.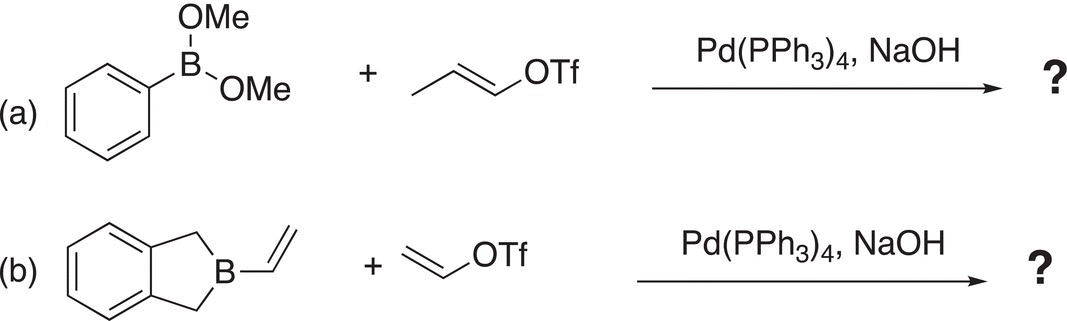
ii. Give the reactants to complete the Suzuki reactions.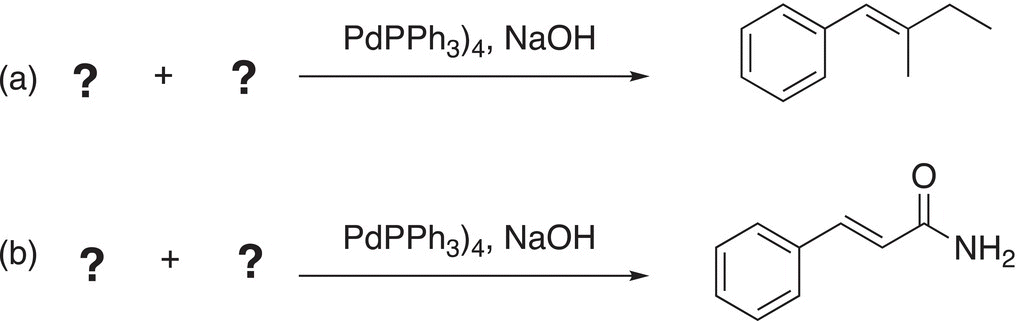
19.3.3 The Stille Coupling Reaction
This reaction, named after John Stille of Colorado State University, is another method of synthesizing new carbon—carbon bonds to make larger molecules from smaller molecules. The general reaction scheme is shown in Reaction (19-22).
(19-22)
A specific reaction is shown below. Note that this reaction essentially couples two benzene rings, a very difficult task to accomplish as we have seen using the chemistry described in the earlier chapters of this book.
(19-23)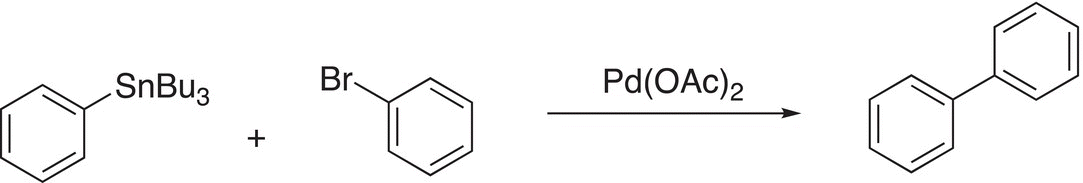
A summary of the Stille reaction is given in Figure 19.3.
Problem 19.4
i. Give the major organic products to complete the Stille coupling reactions.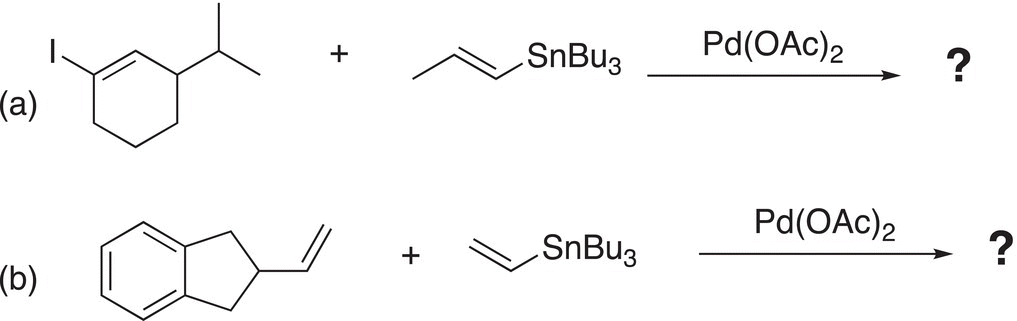
ii. Give the reactants to complete the Stille coupling reactions.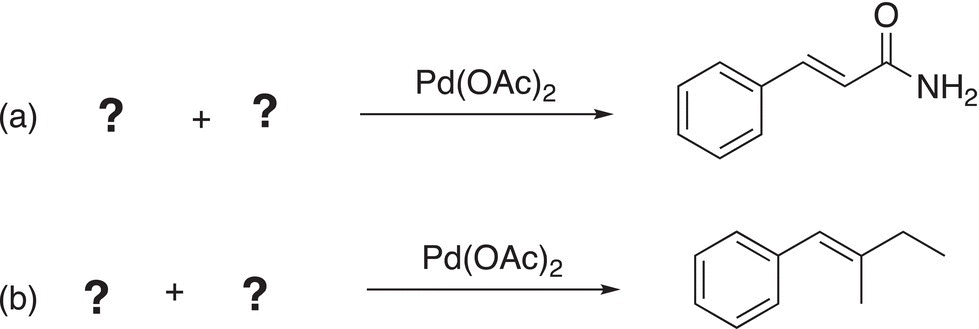
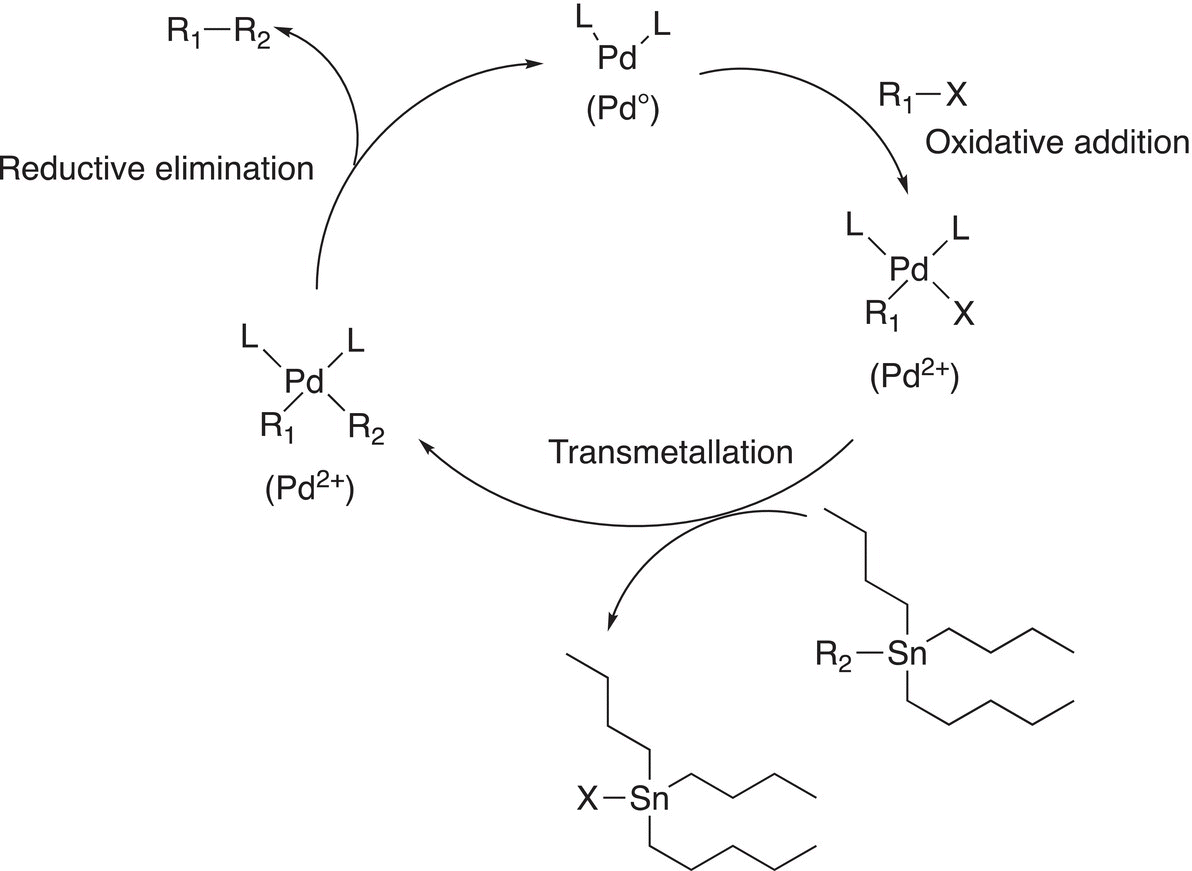
Figure 19.3 A summary of the Stille catalytic coupling reactions.
19.3.4 The Negishi Coupling Reaction
The last reaction that will be covered is the Negishi coupling reaction. This reaction is named after Ei-ichi Negishi, a Japanese chemist, who carried out most of his research work at Purdue University and shared the Nobel Prize in Chemistry in 2010 with Heck and Suzuki for the discovery of the palladium-catalyzed cross-coupling reactions in organic synthesis. The general reaction is shown in Reaction (19-24).
(19-24)
A summary of the Negishi palladium-catalyzed cross couplings is given in Figure 19.4.
Problem 19.5
Give the reactants to complete the Negishi cross-coupling reactions.
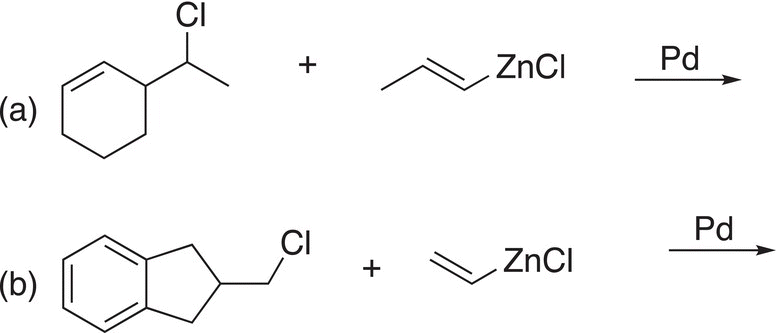
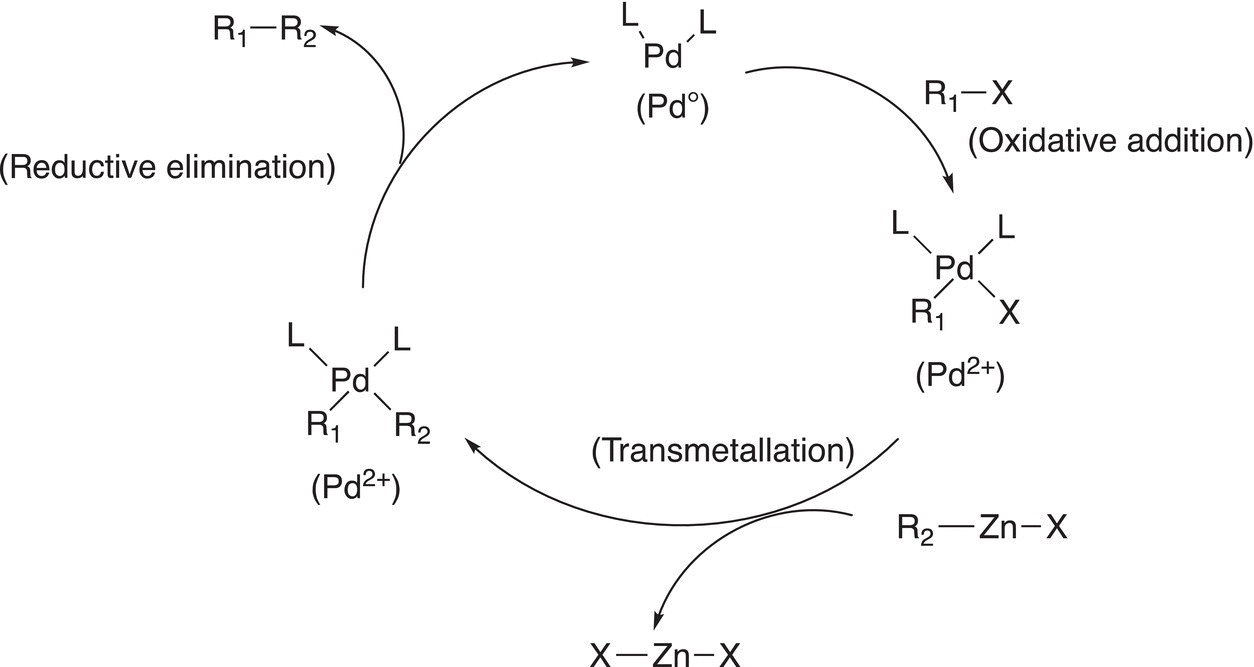
Figure 19.4 A summary of the Negishi catalytic coupling reactions.