Organic Chemistry: Concepts and Applications - Headley Allan D. 2020
Synthetic Polymers and Biopolymers
20.2 Cationic Polymerization of Alkenes
In this section, we will take advantage of the knowledge we gained from studying addition reactions involving alkenes that were covered in Chapter 8. As we saw, different reaction paths can be achieved depending on the type of reagent added to alkenes. For example, in the addition of an electrophile to alkenes, the intermediates that result are carbocations; the addition of a nucleophile to alkenes results in carboanions, and the addition of radicals to alkenes results in radical intermediates. The intermediates that are generated from an initial reaction of a reagent as described above with an alkene are reactive and can react with the initial alkene reactant alkene to form polymeric compounds. In the next sections, we will examine possible reactions of alkenes using different types of reactants.
Table 20.1 Selected polymers produced from various monomers through polymerization.
Monomer |
Polymer |
Properties |
Uses |
CH2═CH2 |
Polyethylene |
Flexible |
Plastic containers |
CH2═CH─Cl |
Polyvinyl (PVC) |
Rigid |
Plumbing pipes |
Ph─CH═CH2 |
Polystyrene |
Semi-rigid |
Containers and packing materials |
CF2═CF2 |
Polytetrafluoroethylene (Teflon) |
Extremely high melting point |
Engine gaskets |
CH2═C(CH3)CO2CH3 |
Poly(methylmethacrylate) |
Flexible, clear |
Lenses, windows |
CH2═CH─CN |
Polyacrylonitrile |
Strong, crystalline |
Fibers |
20.2.1 Cationic Polymerization of Isobutene
We have seen from previous chapters that alkenes in the presence of a Lewis acid will form carbocations, which are good electrophiles and can be attacked by a nucleophilic alkene to form another carbocation, as shown in Reaction (20-2).
(20-2)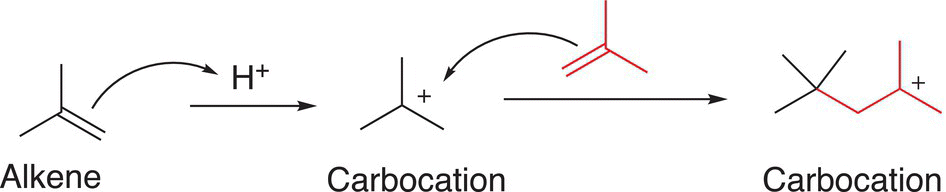
As you can imagine, in the presence of an excess of an alkene, this process will continue as illustrated in Reaction (20-3).
(20-3)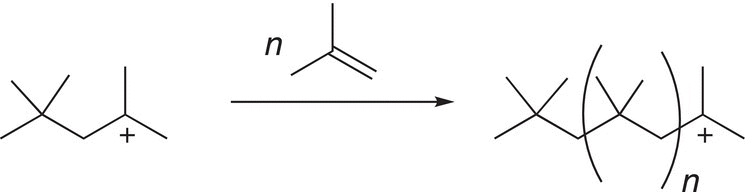
As we have learned in previous chapters, a carbocation can lose a proton to form an alkene. This polymerization of 2-methylpropene, also known as isobutene, can be terminated by loss of a proton, as shown in Reaction (20-4).
(20-4)
Polymerization of isobutene is used in the manufacture of rubber.
20.2.2 Cationic Polymerization of Styrene
Polystyrene is used in a vast majority of household containers and packing materials. The starting material for polystyrene, as the name suggests, is styrene. Note that styrene has a carbon—carbon double bond feature as the alkene used above, and hence the addition can take place in two possible ways to produce two different carbocation intermediates. As shown in Reaction ,(20-5) one carbocation is more stable than the other.
(20-5)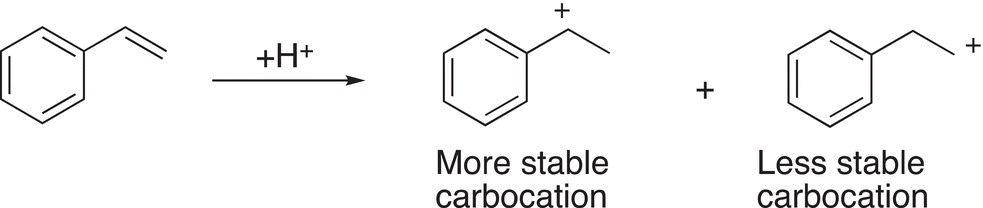
In the next step of the reaction, the carbocation is attacked by another mole of the alkene to generate two different additional carbocations, as shown below in Reaction (20-6). Again, one is more stable than the other carbocation.
(20-6)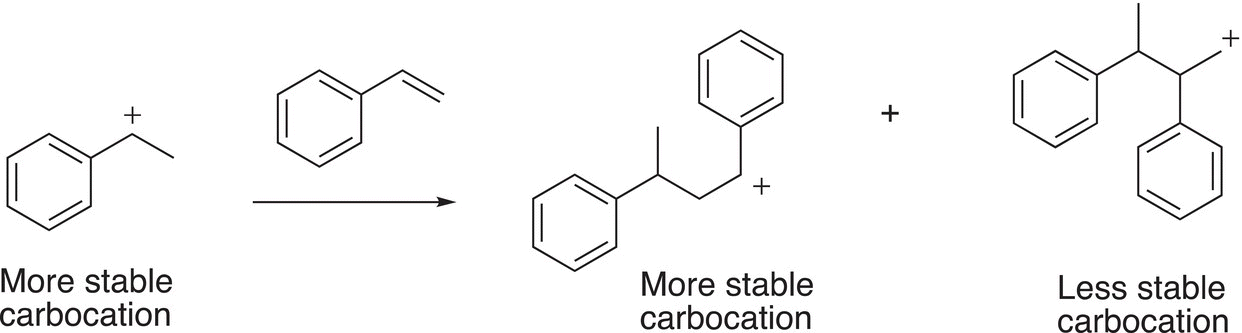
In the above reaction, the more stable carbocation is again the benzylic carbocation. The reaction in which the least stable carbocation reacts with another mole of styrene is shown in Reaction (20-7).
(20-7)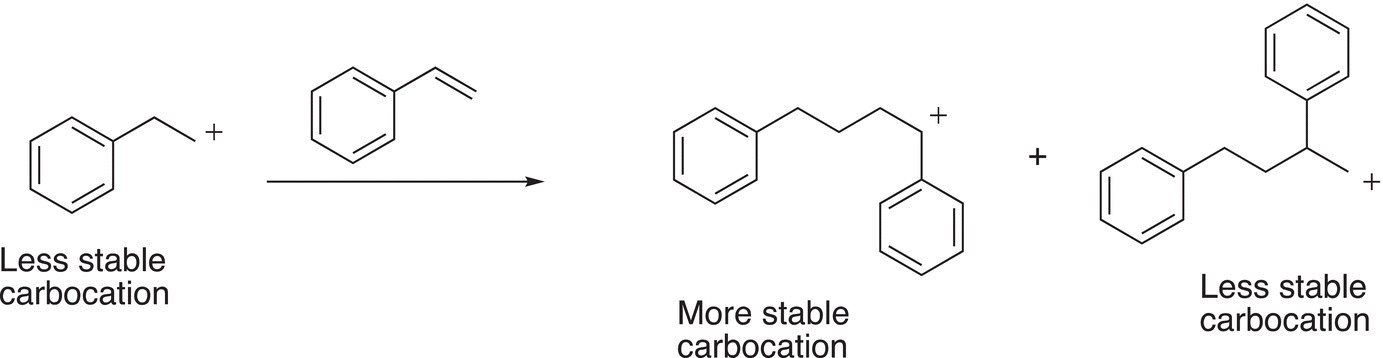
Note that this coupling results in a different set of carbocations, compared to the coupling reaction given in Reaction 20-6. The coupling of the stable benzylic carbocation with styrene to generate polystyrene is shown below.
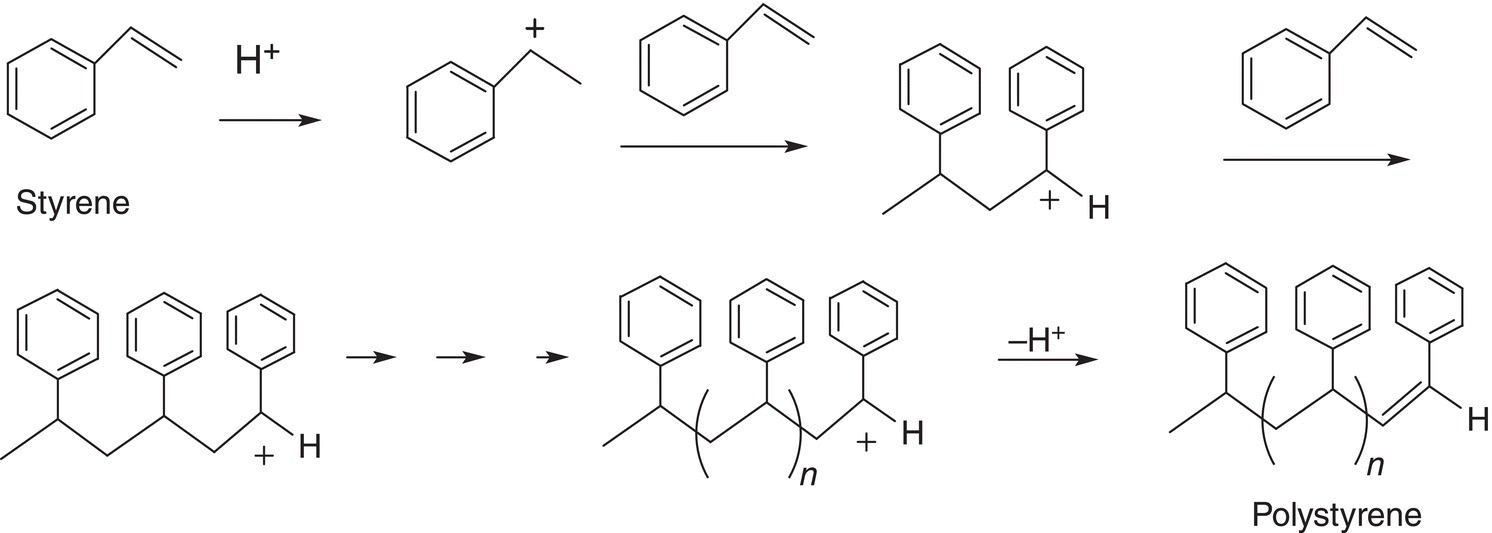
This process results in polystyrene as the product, and polystyrene is one of the most useful polymers in everyday life. Polystyrene is a clear, brittle plastic that is used for transparent containers, insulation, and even inexpensive lenses.
Problem 20.1
i. Give the polymer that would result from the polymerization of ethylene (CH2═CH2), which is also called polyethylene and used as thin plastic wrap.
ii. Give the polymer that would result from the polymerization of tetrafluoro ethylene (CF2═CF2), which is also known as Teflon.