Organic Chemistry: Concepts and Applications - Headley Allan D. 2020
Alkanes, Cycloalkanes, and Alkenes: Isomers, Conformations, and Stabilities
4.4 Conformational Isomers of Cycloalkanes
Free rotation about carbon—carbon bonds in cycloalkanes is not possible. There is the possibility of very limited internal rotation about any carbon—carbon bond in a cyclic molecule, however. Of course, for alkanes with small rings, internal bond rotation is very limited, compared to cycloalkanes with larger rings. In this section, we will examine the various conformations brought about by the limited internal rotation of carbon—carbon single bonds of cycloalkanes.
4.4.1 Isomers of Cyclopropane
For cycloalkanes that are smaller than the cyclopentane and cyclohexane molecules, there is very limited internal rotation about any carbon—carbon single bond. As a result, groups that are bonded to any of the carbons of cyclopropane are either on the same side of the ring or on opposite sides of the ring. The different location of groups relative to the fixed ring structure results in different isomers, called geometric isomers. Figure 4.8 shows two different representations of the arrangements of groups about the rigid cyclopropane ring. If two groups are on the same side of the ring, the relationship is a cis and the isomer is called a cis isomer. If the groups are on opposite sides of the rigid ring structure, the isomer that results is called a trans isomer.
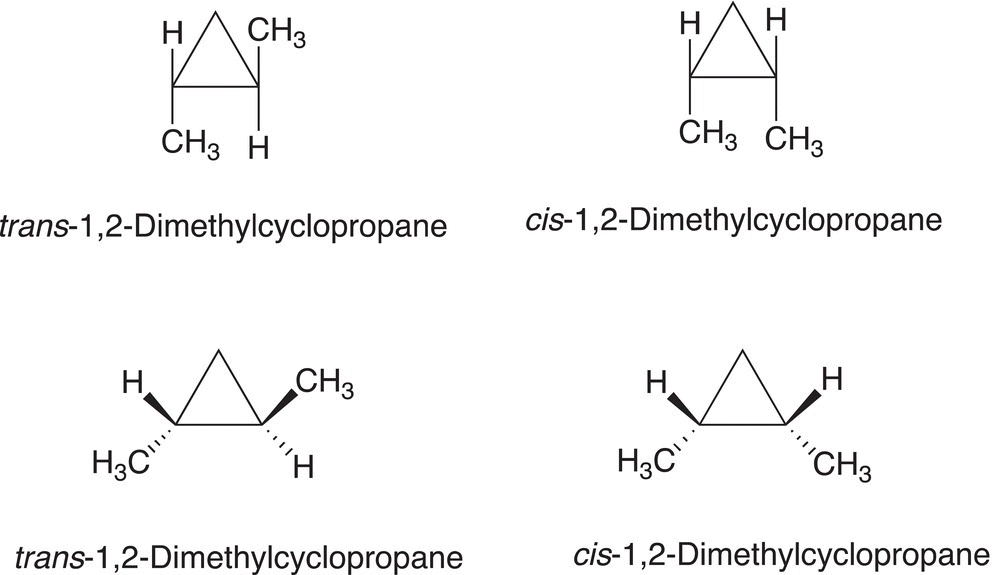
Figure 4.8 Representations of trans and cis arrangements of 1,2-dimethylcyclopropane.
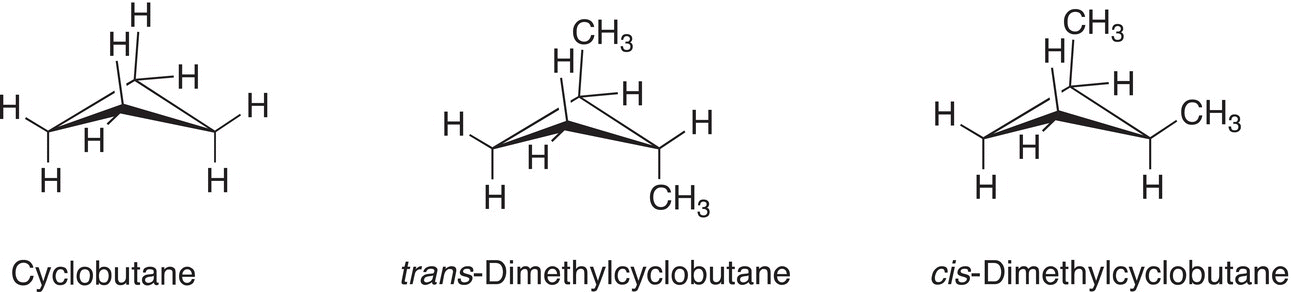
Figure 4.9 Representations of cyclobutane and trans and cis-dimethylcyclobutane.
4.4.2 Conformational Isomers of Cyclobutane
Limited internal rotation about the carbon—carbon bonds of cyclobutane is just slightly greater than that of cyclopropane. The cyclobutane ring is considered to be rigid and as a result, hydrogens and substituents bonded to carbons of cyclobutane can be either on the same side of the fairly rigid cyclobutane ring or on opposite sides as shown in Figure 4.9.
Compared to cyclopropane, the cyclobutane ring is not as flat and sometimes described as puckered as illustrated in Figure 4.9.
Problem 4.7
i. Draw the cis and trans isomers of 1,2-dibromocyclobutane.
ii. Label the following as cis or trans 1,2-dibromocyclobutane.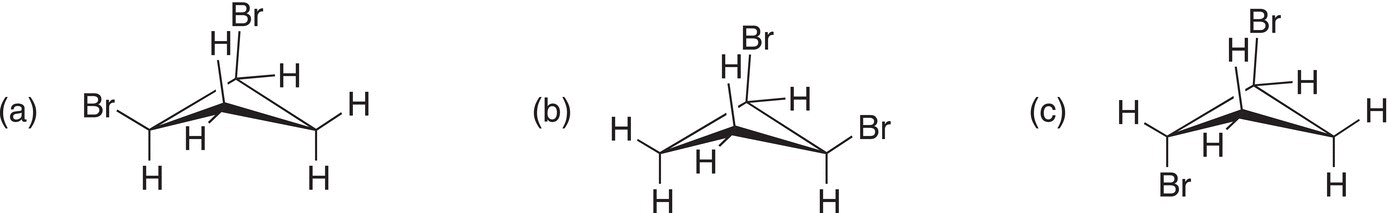
iii. Draw the cis and trans isomers of 1,2-dimethylcyclobutane.
4.4.3 Conformational Isomers of Cyclopentane
The increased size of cyclopentane ring, compared to cyclobutane and cyclopropane rings, introduces greater internal rotation about the carbon—carbon single bonds. For the cyclopentane ring, the hydrogens can be described based on their orientation relative to the plane of the cyclopentane ring. A close examination of the hydrogens bonded to the cyclopentane ring reveals that five hydrogens (one of each carbon) appear to be almost in the same plane as the ring, and as a result, they are described as equatorial hydrogens. The other five hydrogen atoms (one on each carbon) appear to be either above or below the plane of the cylopentane ring and they are described as axial. This observation is illustrated in Figure 4.10, where Ha represents axial hydrogens and He represents equatorial hydrogens.
As a result, the relationship between any two substituents on the cyclopentane ring can be described in two ways: cis and trans or based on their location as axial or equatorial. An example of the two conformers of 1,2-dimethylcyclopentane is shown in Figure 4.11.
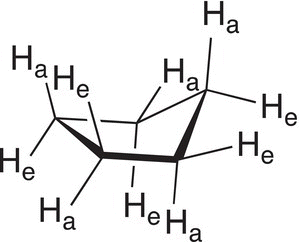
Figure 4.10 Cyclopentane showing axial hydrogens (Ha) and equatorial hydrogens (He).
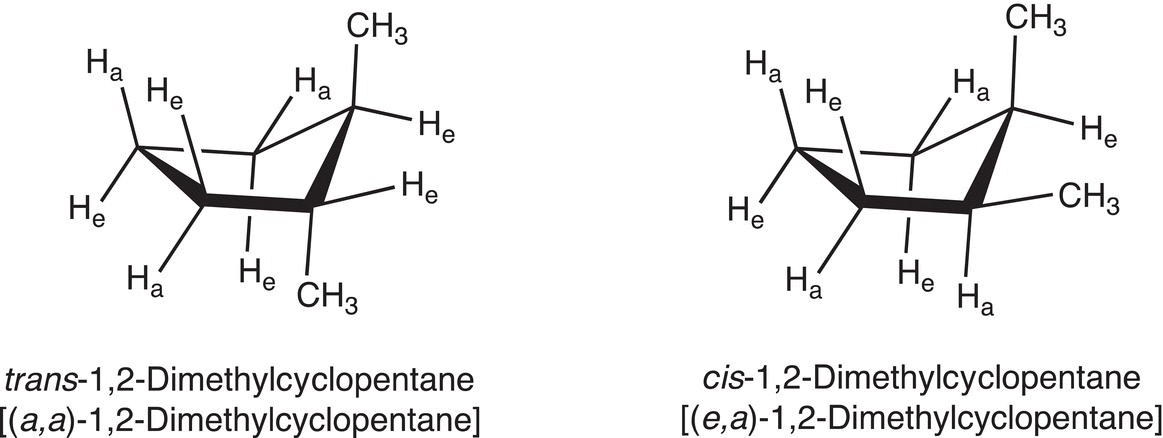
Figure 4.11 cis and trans-Dimethylcyclopentanes.
The possibility of different conformers of 1,3-disubstituted cyclopentane also exists, and Figure 4.12 shows two conformers of 1,3-dichlorocyclopentane, the cis and trans conformers.
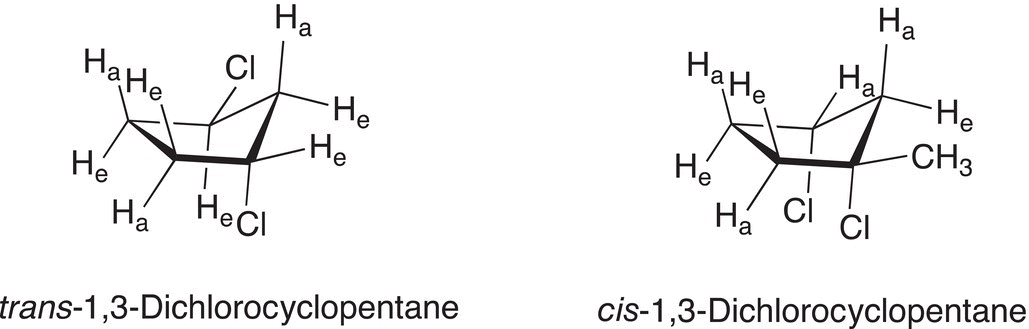
Figure 4.12 Different conformations of cis and trans-1,3-dichlorocyclopentane.
Problem 4.8
Draw the trans conformers of the following molecules.
1. 1,2-Dibromocyclopentane
2. 1,3-Dibromocyclopentane
Since there is greater internal carbon—carbon rotation within cyclopentane, compared to that of cyclopropane and cyclobutane, the difference between the cis and trans conformers of cyclopentane is not as obvious, compared to cis and trans conformers of disubstituted cyclopropane and cyclobutane.
4.4.4 Conformational Isomers of Cyclohexane
There is more internal carbon—carbon bond rotation within cyclohexane than cyclopentane, which leads to a more obvious difference in the conformers, compared to cyclic structures discussed earlier. One of the first observations about cyclohexane is that it can acquire one of two different conformations based on greater internal bond rotation around the carbon—carbon single bonds, of which two are shown in Figure 4.13. The conformer shown on the left looks like a chair, hence it is called the chair conformer. The other conformer on the right looks like a boat, and hence it is called the boat conformer.
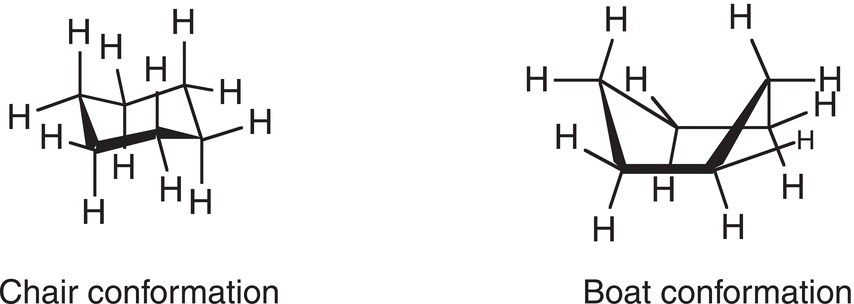
Figure 4.13 Chair and boat conformations of cyclohexane.
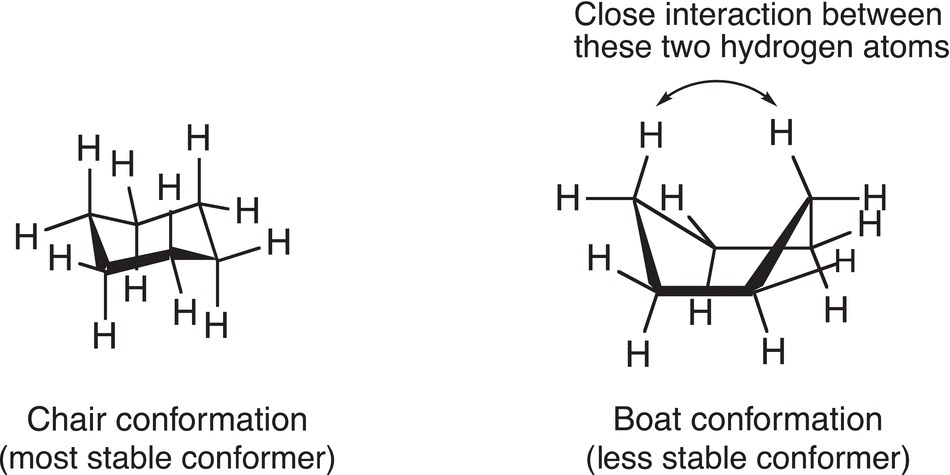
Figure 4.14 Illustration of the rationale for the relative stabilities of the chair and boat conformers of cyclohexane.
Of these two conformers, the chair conformer is the most stable conformer. The boat conformer is less stable because the hydrogens are closer to each other, especially those in the 1,4 positions as illustrated in Figure 4.14.
Since the chair conformer is the most stable conformer, it will be the conformer that will be analyzed in details relative to the boat conformer, which is less stable. A close examination of the chair conformer of cyclohexane reveals that there are six hydrogens (one on each carbon) that are essentially in the same plane as the six carbon atoms; as a result, they are described as equatorial (e). Likewise, there are six hydrogens (one on each carbon) that are above and below the plane of the cyclohexane ring and these hydrogens are described as axial (a). In fact, three hydrogens of these hydrogens are above the plane and three are below the plane. This observation is illustrated in Figure 4.15.
At room temperature, the cyclohexane molecule is in equilibrium with both conformations, but the equilibrium favors the stable chair conformer, compared to the boat conformer. That is, the molecule is constantly flipping from one chair conformation to another chair through the boat conformation, as shown in Figure 4.16. The energy required to “flip” from one cyclohexane conformer to another conformer through the boat conformer is very small. Thus, a flip from one chair conformation to another is accomplished easily at room temperature.
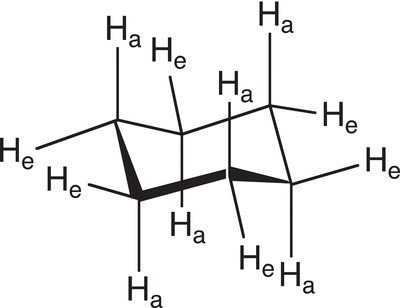
Figure 4.15 Representation of the different hydrogens of cyclohexane chair conformation. He represents hydrogens in the equatorial position, and Ha represents hydrogens in the axial position.
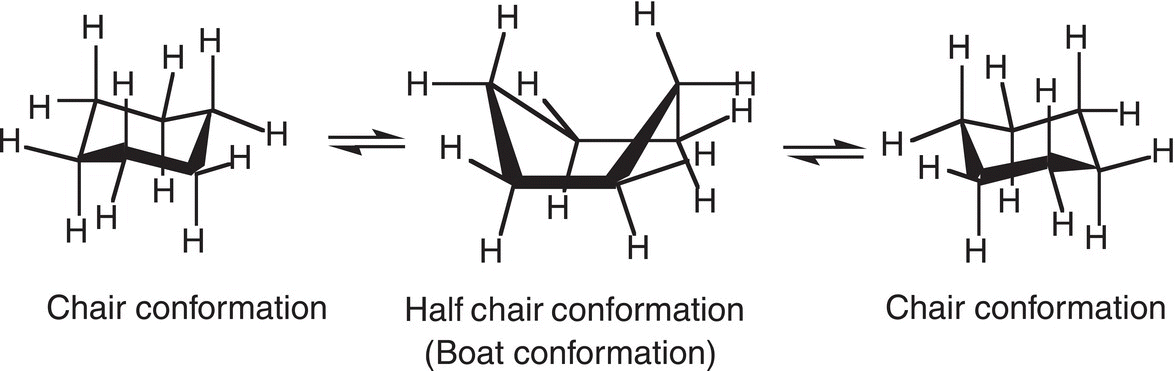
Figure 4.16 Conformational changes of cyclohexane from one chair conformer to another through the boat conformation.
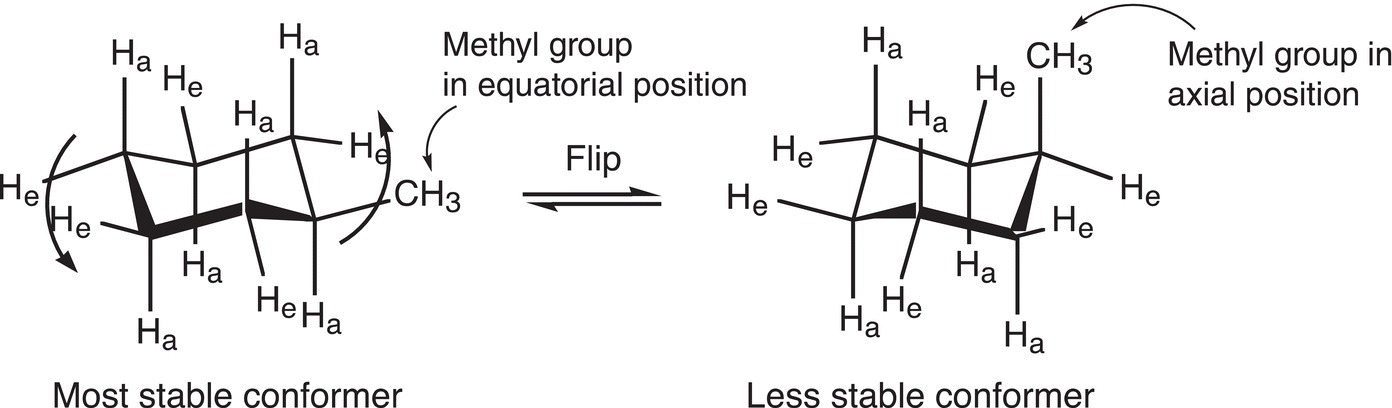
Figure 4.17 Two chair conformers of methylcyclohexane.
4.4.5 Conformational Isomers of Monosubstituted Cyclohexane
Whenever there is a ring flip for cyclohexane, there is no noticeable change between both conformers. If one of the hydrogens of cyclohexane is substituted for a methyl group, to produce methylcyclohexane, then the methyl group can occupy either the axial or the equatorial position, and there is a noticeable difference in the location of the methyl group between both chair conformers when there is a ring flip. Consider the ring flip of methylcyclohexane as shown in Figure 4.17
For the chair conformer to the left, the methyl group is in the equatorial position and after the ring flip to form the other chair conformation, the methyl group is in the axial position (conformer on the right). A close examination of these two conformers reveals that the larger methyl group (compared to the hydrogens) would much prefer to occupy the equatorial position since there is more space in the equatorial plane to accommodate a large group, compared to the axial plane. For these conformers, the chair conformer to the left is more stable than the conformer shown on the right. The greater stability of the conformation on the left comes from the methyl group being in the equatorial position, where there is more space, compared to the axial. The other conformer (the one to the right) is the least stable conformer since the methyl group is in the axial position where there are more steric interactions with the other axial hydrogens and the methyl group.
If larger groups such as the 1,1-dimethylethyl [─C(CH3)3, tert-butyl] are placed on the cyclohexane ring, the energy difference between the two conformers would be greater, compared to the energy difference for methylcyclohexane. As a result, the conformer which has the tert-butyl group in the equatorial position would be the preferred conformer, compared to the conformer in which the group is in the axial position.
Problem 4.9
1. Draw the most stable conformer of chlorocyclohexane.
2. Draw the least stable conformer of bromocyclocyclohexane.
4.4.6 Conformational Isomers of Disubstituted Cyclohexane
Disubstituted cyclohexanes, such as 1,4-dimethylcyclohexane, have more isomers and hence conformational isomers, compared to monosubstituted cyclohexane, such as methylcyclohexane, as we have seen in the previous section. Figure 4.18 shows the possible chair conformers of 1,4-dimethylcyclohexane.
Note that since there are now two groups on the cyclohexane ring, the possibility of cis and trans isomers exists for each conformer. Hence, there are four possible isomers for a disubstituted cyclohexane, compared to two conformers for a monosubstituted cyclohexane, as discussed in the previous section. For the first two conformers shown in Figure 4.18, notice that the two methyl substituents are on opposite sides of the plane of the cyclohexane ring, hence they are trans isomers. The methyl groups of the first conformer of this equilibrium are at the opposite ends of the cyclohexane ring and for the other conformer of this equilibrium, the methyl groups are above and below the plane of the cyclohexane ring. As a result, they are described a trans conformers. Thus, if the relationship between two groups in the 1,4 positions of the cyclohexane ring is in the equatorial-equatorial locations, or axial-axial locations, the isomers are trans isomers.
For the second equilibrium shown in Figure 4.18, notice that the two methyl substituents are not on the opposite sides of the plane of the cyclohexane ring. This is a bit more difficult to visualize, but in the first case, they are axial-equatorial, which gives a cis relationship; for the second conformer, the groups are also axial-equatorial, which also gives a cis relationship. As a result, these two conformers are described cis conformers.
Problem 4.10
1. Figure 4.18 shows four conformers of 1,4-dimethylcyclohexane, which is the least stable conformer and explain your answer?
2. Draw most stable conformer of 1,4-dichlorocyclohexane.
Another representation of the conformers shown in Figure 4.18 is shown in Figure 4.19, in which the dashed-wedge representation is used. Note that with this type of representation, the axial and equatorial positions cannot be shown. This representation just gives the relationship between the groups bonded to the ring.
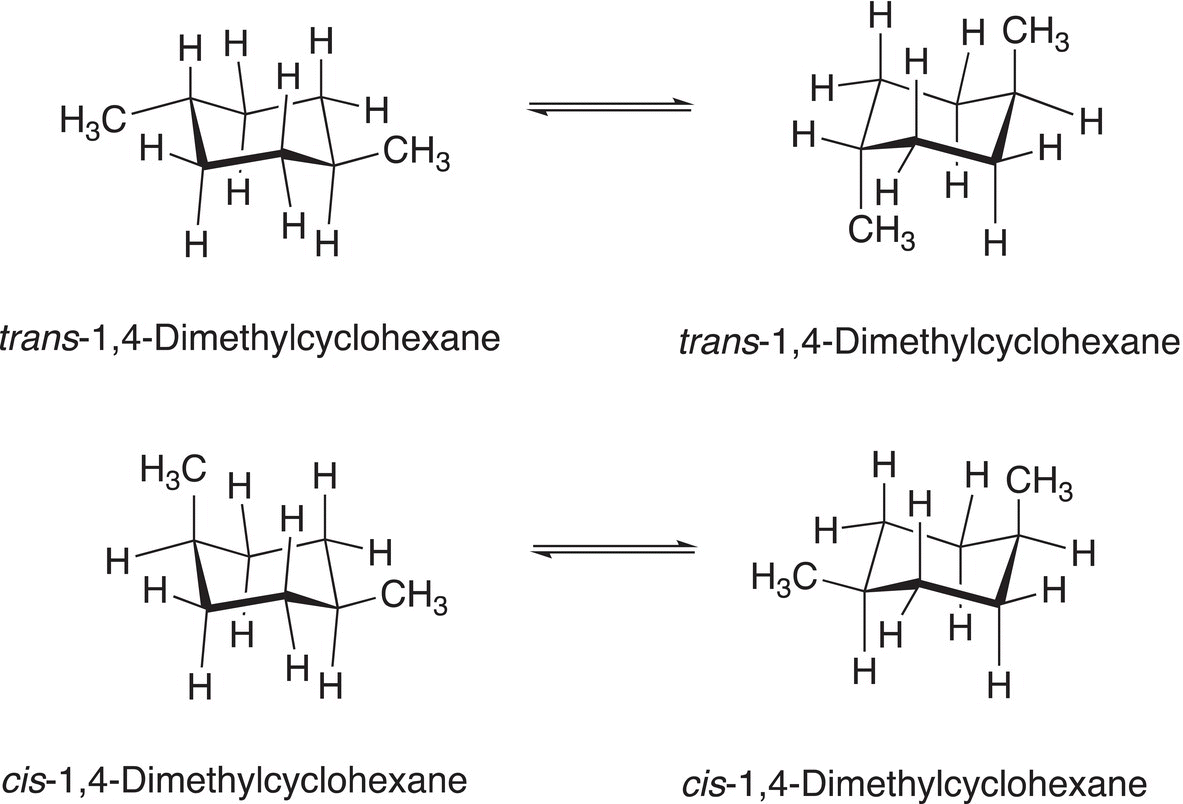
Figure 4.18 Possible conformers of 1,4-dimethylcyclohexane.
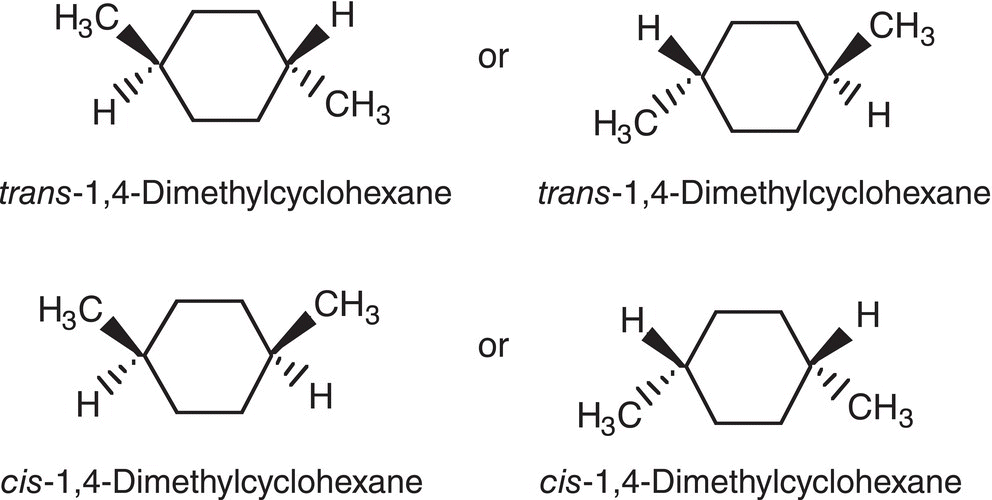
Figure 4.19 Possible conformers of 1,4-dimethylcyclohexane in which the dashed-wedge representation is used.
Table 4.1 Conformers of cis and trans isomers of disubstituted cyclohexanes.
Relationship |
Cis |
Trans |
1,2 |
a,e or e,a |
e,e or a,a |
1,3 |
a,a or e,e |
a,e or e,a |
1,4 |
a,e or e,a |
a,a or e,e |
For other disubstituted cyclohexanes, such as 1,3-dimethylcyclohexane and 1,2-dimethylcyclohexane, a similar analysis can be carried out. For these compounds, it is possible to have different cis and trans conformers similar to those shown in Figure 4.18. Table 4.1 gives a summary of the types of isomers, based on the arrangements of the groups in the equatorial or axial positions of the cyclohexane ring. At this point, a model set would be very helpful to better visualize the relationships for the different conformers.
Problem 4.11
1. Draw a chair conformer of trans-1,2-dimethylcyclohexane.
2. Draw the chair conformer of the most stable conformer of 1,2-dimethylcyclohexane.
3. Draw a chair conformer of cis-1,3-dimethylcyclohexane.