Organic Chemistry: Concepts and Applications - Headley Allan D. 2020
Alkanes, Cycloalkanes, and Alkenes: Isomers, Conformations, and Stabilities
4.5 Geometric Isomers
For compounds that have a double bond, there can be different arrangements in space of the atoms or groups bonded to the carbons of the double bond. There is no rotation about a carbon—carbon double bond like that of a carbon—carbon single bond. Due to the lack of rotation about a carbon—carbon double bond, it is impossible to have conformational isomers, but it is possible to have different groups on the same side of the double bond, or on opposite side of the carbon—carbon double bond. Geometric isomers are molecules that have different spatial atomic arrangements based on a rigid framework in the molecule, such as a double bond or a rigid cyclic structure as demonstrated with the cyclopropane ring, which was discussed earlier in the chapter and illustrated in Figure 4.8. The terms cis and trans are used to describe the spatial arrangement of two atoms or groups about a rigid system, such as a double bond. The cis geometric stereoisomer has the same atoms or groups on the same side of the double bond, and the trans stereoisomer has the same atoms or groups on the opposite side of the double bond. 1,2-Difluoroethene has two different geometric isomers, which have the same atom-to-atom connectivity, yet the dipole moments (μ) of these molecules are different; the same is true for cis and trans 2-butene as shown in Figure 4.20.
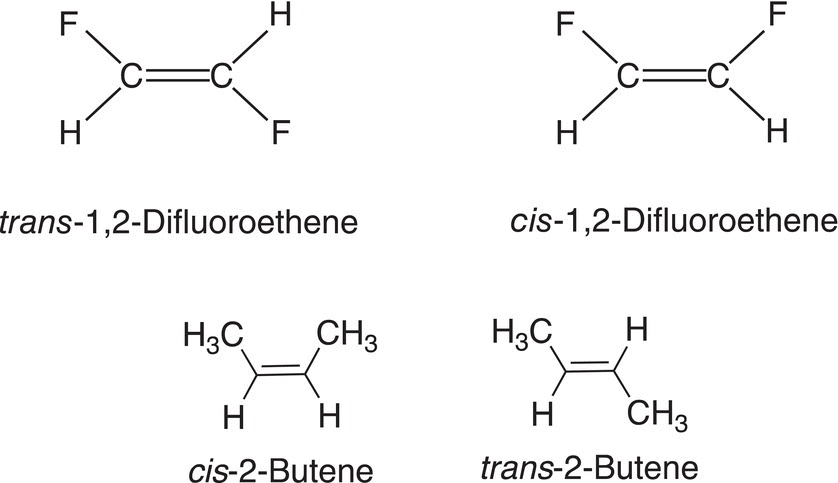
Figure 4.20 Different isomers (geometric isomers) of 1,2-difluoroethene and 2-butene.
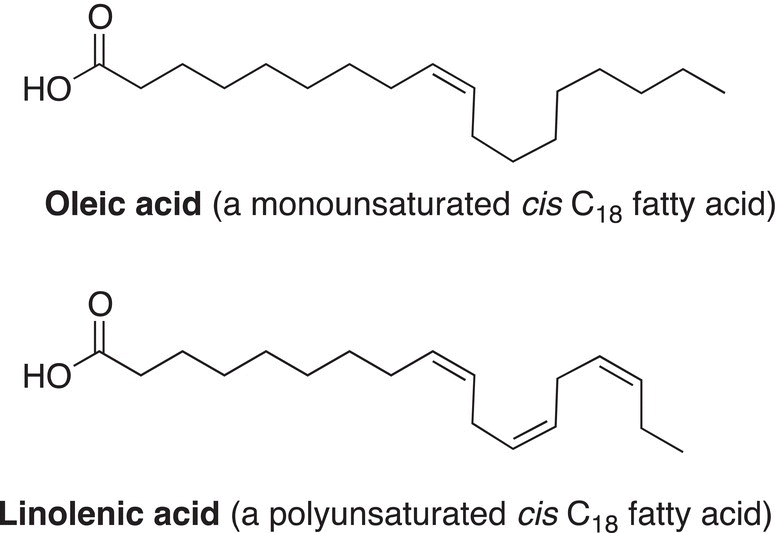
Figure 4.21 Examples of fatty acids (unsaturated fatty acids with cis double bonds).
The term unsaturation is sometimes used to describe the presence of the carbon—carbon double bonds in molecules. For example, unsaturated fats have at least one double bond (site of unsaturation), and as a result, it is possible to have geometric isomers about the double bonds of unsaturated fats, as shown in the examples in Figure 4.21. Due to the presence of trans and cis double bonds in fatty acids, the melting points of these compounds are typically lower than their unsaturated counterpart. Unsaturated fatty acids, like the ones shown in Figure 4.21, cannot fit into a nicely defined crystal structure, compared to saturated fatty acids; as a result, they are typically liquids at room temperature, compared to saturated fatty acids that are typically solids at room temperature.
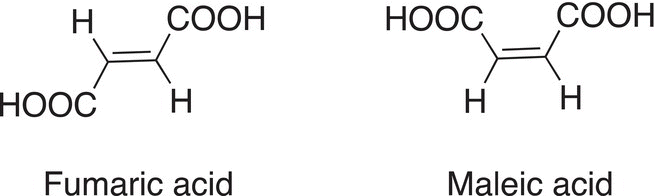
Another example of the differences in properties brought about due to different geometric isomers is that of fumaric acid and maleic acid. As shown below, these compounds are geometric isomers and the body recognizes one as essential for health, whereas the other is toxic; the only difference between both molecules is the orientation of the groups in space across a rigid double bond, they are geometric isomers of each other.
4.5.1 IUPAC Nomenclature of Alkene Geometric Stereoisomers
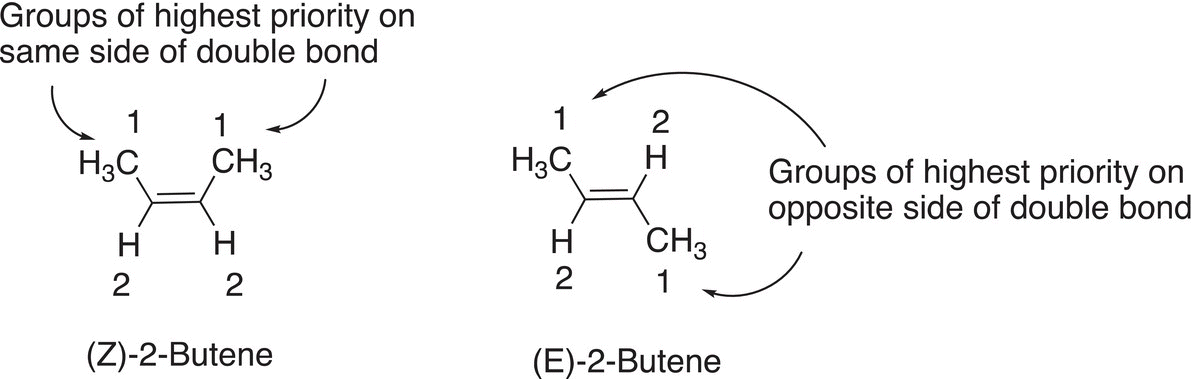
Owing to the difference in properties of geometric isomers arising from orientations of groups about the double bond, it is essential to be able to appropriately identify different geometric isomers. Since geometric stereoisomers are obviously different molecules; as a result, they must have different names. Cis and trans are common descriptions of such molecules, but another system, the Z and E symbolism is used in the IUPAC system of nomenclature. The designations Z and E are derived from the German terms zusammen, which means together and entgegen, which means opposite. Hence the Z and E are used to designate the relationship of the groups of highest priority about the carbon—carbon double bond. The priority of a group is determined based on the atomic number of the atoms that are directly bonded to the carbons of the alkene double bond. If an atom that is bonded directly to the carbon of the double bond has a larger atomic number than another atom that is bonded to the same carbon of the double bond, the atom with the larger atomic number is assigned the highest priority. If the atoms or groups of highest priority are on the same side of the double bond, the letter Z is assigned to describe that arrangement. The letter E is assigned if the groups of highest priority are on opposite sides of the double bond. The E and Z system of naming is typically used for alkenes, and the trans and cis descriptions are typically used for cyclic compounds that have different geometric stereoisomers.
An example of the use of the E and Z system is demonstrated in naming the geometric isomers of 1,2-difluoroethene, which is shown in Figure 4.22. In this figure, the fluorine atom has the highest atomic number, compared to the hydrogen atom; hence, fluorine gets the higher priority. Thus, for the geometric isomer on the left, the groups of highest priority are on opposite side of the double bond and the designation for that geometric isomer is the (E) isomer. The geometric isomer on the right has the groups of highest priority of the same side and is designated the (Z) geometric isomer.
Shown below is the application of the E and Z system for naming the geometric isomers of 2-butene; the highest priority group (or atom) is assigned #1.
For more complex molecules where the groups bonded to the carbon—carbon double bond are completely different, a careful examination of the atomic numbers of each atom bonded to the carbons of the double bond must be carried out. Shown in the examples below are more complex molecules, in which a methyl group, a hydrogen, and two different halogens are bonded to the carbons of the double bond. For the first example, the carbon on the left of the double bond has two groups bonded, a bromine atom and a chlorine atom. Since the atomic number of the bromine is greater than that of chlorine, bromine is assigned the highest priority and gets #1 and chlorine gets #2. For the other carbon of the double bond, there is a methyl group and a hydrogen bonded to that carbon. Since the carbon, which is directly bonded to the carbon of the double bond, has a larger atomic number than that of hydrogen, the methyl group has the highest priority and gets assigned #1 and hydrogen #2.
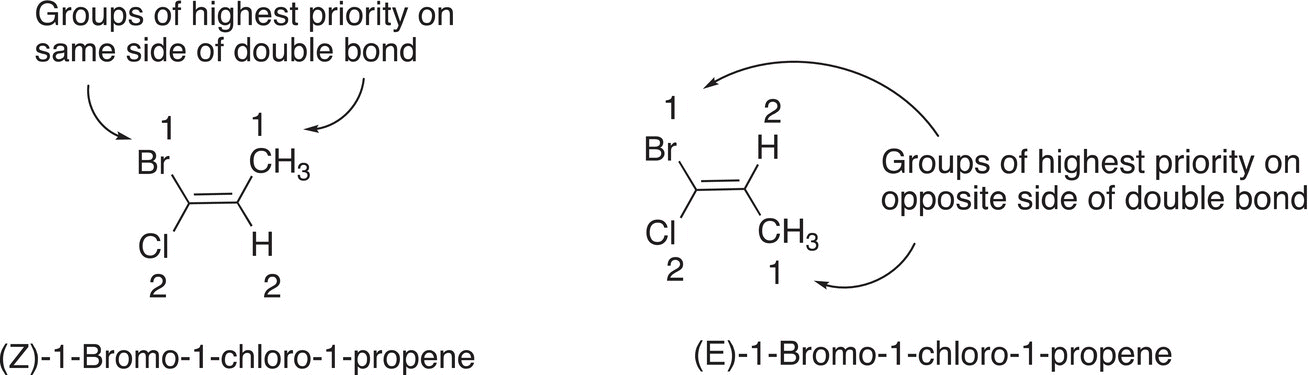
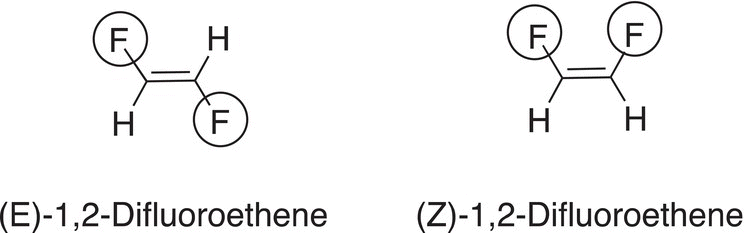
Figure 4.22 E and Z designation in the IUPAC nomenclature system of 1,2-difluoroethene. Atoms with the largest atomic numbers are on opposite side of the double bond. Atoms with the largest atomic numbers are on same side of the double bond.
For this molecule, the groups of highest priority are on the same side, that is, both #1s are on the same side of the double bond. As a result, the geometric isomer is assigned a Z, hence the name shown above. For the other molecule to the right, a similar analysis can be carried out. The carbon on the left of the double bond has a bromine and a chlorine that are bonded directly to the alkene carbon. Hence, the assignment shown is based on the atomic numbers of bromine and chlorine. The same analysis for the right carbon of the double bond gives the methyl group the highest priority and it is assigned #1 and hydrogen #2. Based on this arrangement, the molecule is named as the E geometric isomer.
Problem 4.12
Give the structure of the following molecules.
1. (E)-3,4-Dimethyl-3-heptene
2. (Z)-3-Chloro-4-methyl-4-octene
For most of the molecules that will be encountered in organic chemistry, the determination of the atom, or group, of highest priority is not as obvious as that of the examples discussed so far. As a result, a set of rules must be used to determine the highest priority for the more complex groups.
1. Determine the atomic numbers of the atoms that are directly bonded to the carbon of each of the carbons of the double bond and assign priorities. As mentioned earlier, the atom with the highest atomic number gets priority #1.
2. If there are two isotopes, the isotope with the higher atomic mass is given the higher priority number.
3. If there are two of the same atoms bonded to a carbon of the double bond, consider the atomic number of the next atom bonded to those atoms and use atomic number of the adjacent atom to determine the priority of that group. For example, ─CH2CH3 gets a higher priority, compared to ─CH3.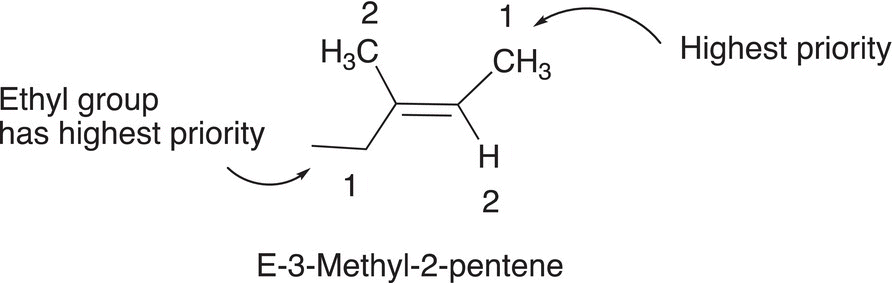
Difunctional molecules discussed in the previous chapter, including molecules that contain the alkene and alcohol functionalities can also exist as geometric isomers as shown in the example below.
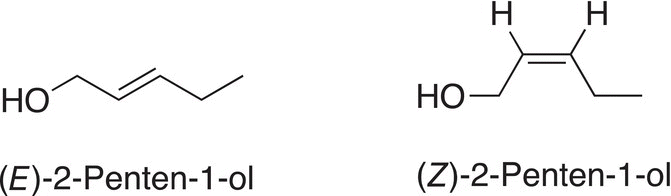
4. For groups that have multiple bonds, the scheme below for the priority assignment is used.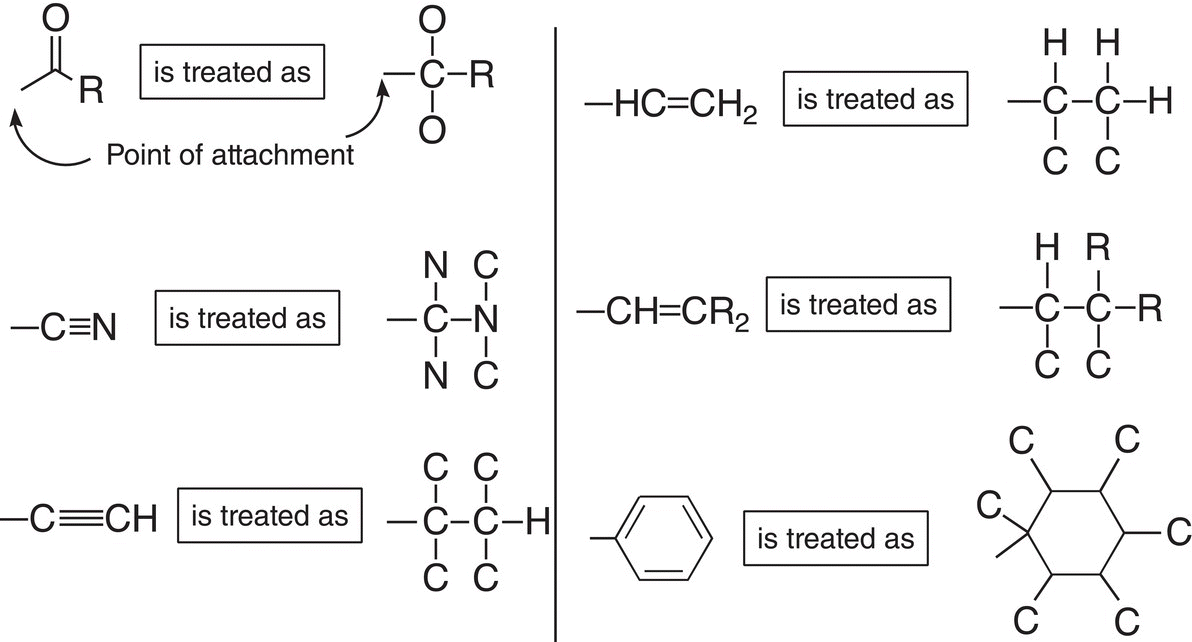
Problem 4.13
1. Of the following pairs of groups, determine which has the highest priority.
1. ─CH3 and ─CH(CH3)2
2. ─CH=CH2 and ─CH3CH3
3. ─CHO and ─OH
4. NH2 and Br.
2. Give the structures and complete IUPAC names (including E and Z description) for the two geometric stereoisomers of 3-methyl-2-pentene.
3. Give the IUPAC for the compounds shown below.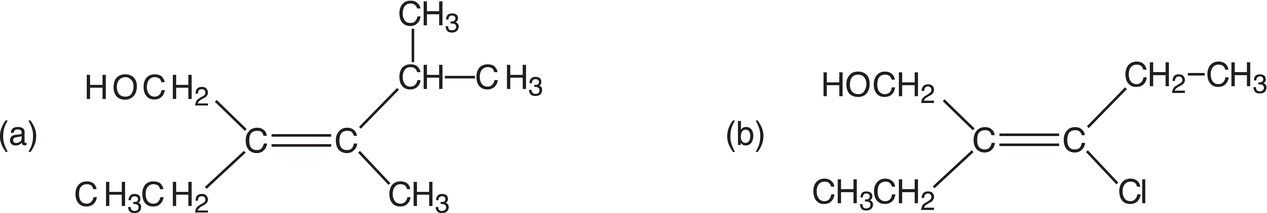
4. Give the IUPAC names for the molecules shown below.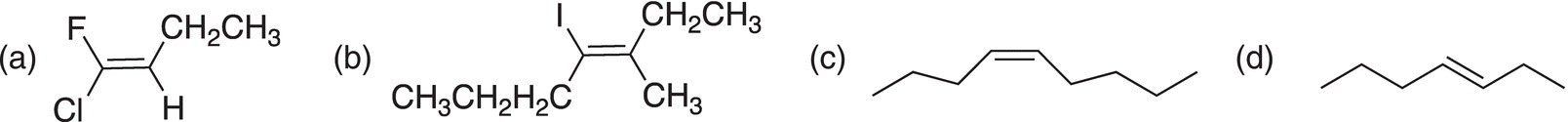
5. Give the line-angle structures for the following molecules.
1. (Z)-1-Chloropropene.
2. (E)-3-Methyl-2-hexene.