Organic Chemistry: Concepts and Applications - Headley Allan D. 2020
Stereochemistry
5.2 Chiral Stereoisomers
As pointed out earlier, some objects and molecules, even though they look very much alike, are not really the same. For example, objects that are mirror images of each other look very much alike, but are they really the same? It is very important to be able to determine subtle differences in the stereochemistry of molecules. A practical way to better understand this concept is to imagine a pair of gloves. They look pretty much the same, but they are really different. The left hand of a pair of gloves will not fit the right hand and vice versa. Thus, one evidence that each glove in a pair of gloves is different from the other is that they are not interchangeable on the right hand and left hand. One test that can be used to determine if two molecules, which look very much alike, are really the same or different is to determine if they are superimposable on each other. For molecules that are superimposable, there is a complete overlay of all atoms in three-dimensional space. If two molecules are superimposable, then they are the same, but if they are not superimposable, then they are not the same — they are different molecules. Molecules in which their mirror images are not superimposable on each other are called chiral or stereogenic molecules. L and D α-amino acids are very similar in appearance, but they are really different molecules since they are not superimposable on each other. Shown in Figure 5.1 are the L and D forms of the α-amino acid, alanine.
Since the mirror image of a stereogenic compound is not the same as the molecule being reflected in the mirror, a stereogenic (or chiral) molecule and its mirror image are really two different compounds, and hence they are isomers, and to be specific, they are stereoisomers. The relationship between such stereoisomers is a special one and is referred to as enantiomerism, and the compounds are called enantiomers. Enantiomers are stereoisomers in which the mirror images of each other are not superimposable on themselves. This concept of enantiomers is illustrated in Figure 5.2.
The molecules shown in Figure 5.2 differ from each other only in the spatial arrangements of the four different groups around the central carbon. This central carbon of compounds that have four different groups is known as a stereogenic carbon. Thus, a stereogenic carbon is a carbon that is bonded to four different groups. An examination of a slightly different molecule, which has the same two atoms or groups bonded to a carbon and two different atoms or groups, gives a totally different outcome, as shown in the molecules in Figure 5.3.
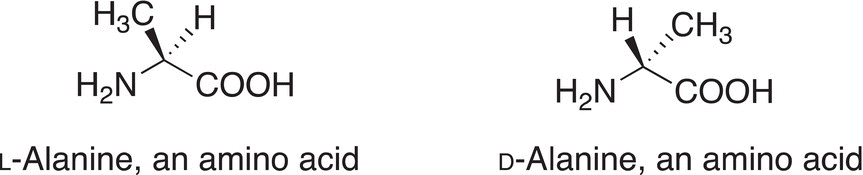
Figure 5.1 L-Alanine and D-alanine are different molecules; they are mirror images of each other and are not superimposable on each other.
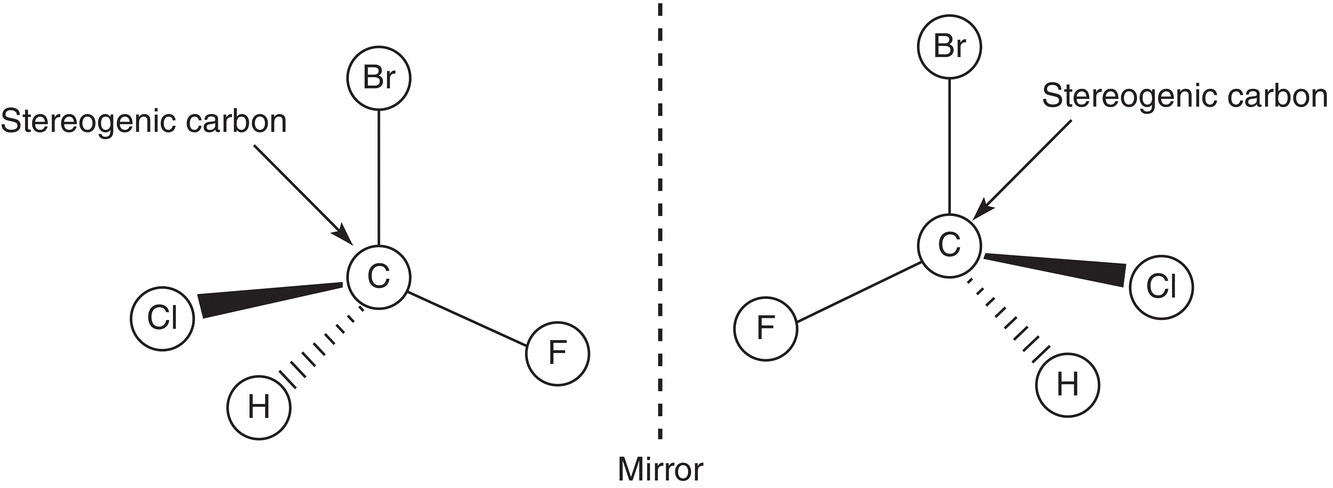
Figure 5.2 Nonsuperimposable mirror images, also known as enantiomers.
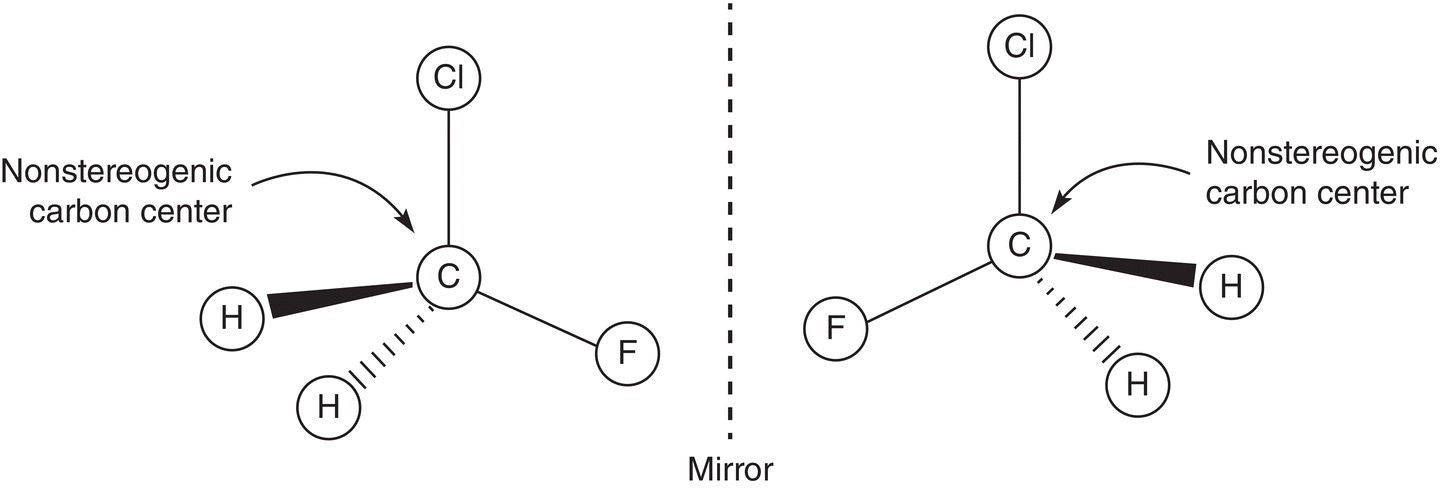
Figure 5.3 Superimposable mirror images, or same molecules.
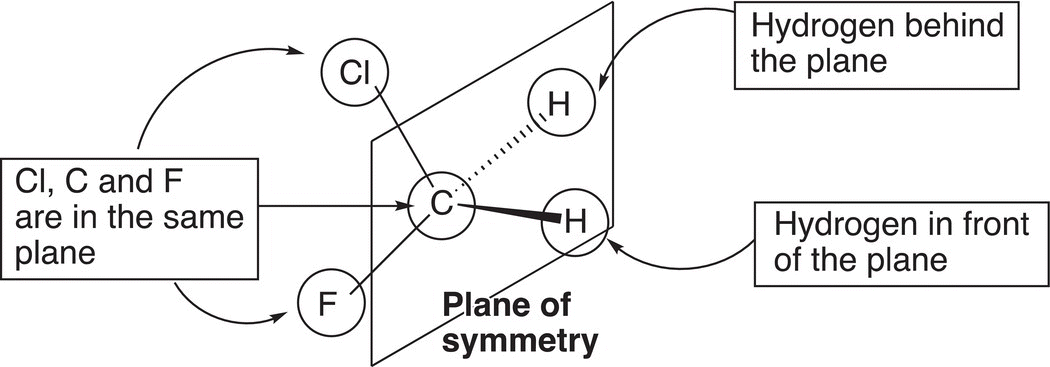
Figure 5.4 An example of a symmetrical molecule, which is not a stereogenic molecule.
Even though these compounds are mirror images of each other, they are superimposable on each other and hence they are not enantiomers, but instead they are the same molecules. Note that for these molecules there is a plane of reflection through the carbon, chlorine, and fluorine atoms, as shown in Figure 5.4. That is, the two hydrogen atoms reflect across that plane. Such molecules are referred to as symmetric molecules or have a plane of symmetry. Such molecules are achiral or not stereogenic.
5.2.1 Determination of Enantiomerism
The use of an imaginary mirror to assist in the determination of enantiomerism is a very tedious exercise. Some simple observations of the molecules in both examples given above can be made to decide if molecules are stereogenic or not. Molecules that are asymmetric or molecules that do not have a plane of symmetry (as the molecules shown in Figure 5.2) are stereogenic and will exhibit enantiomerism. If a molecule has a carbon atom that has four different groups bonded to that carbon, then that molecule is stereogenic. These simple observations will assist in a quick determination of enantiomerism in molecules. Figure 5.5 gives examples of molecules that are stereogenic because they contain one atom (the starred atom) that has four different groups that are bonded to that carbon. Note that for the achiral molecules, there is not a carbon present that has four different groups.
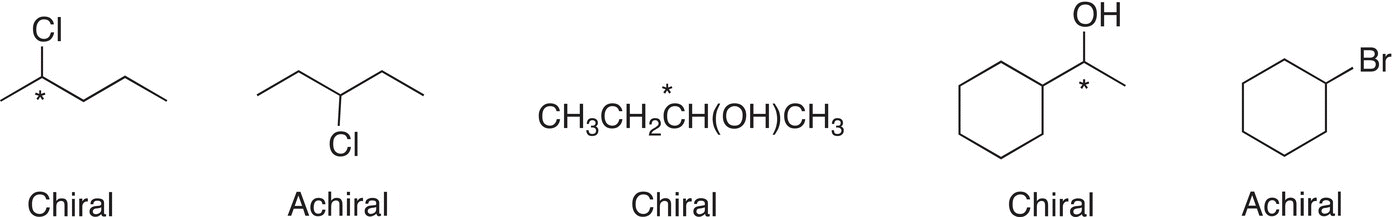
Figure 5.5 Examples of chiral and achiral molecules, an asterisk indicates the stereogenic carbon.
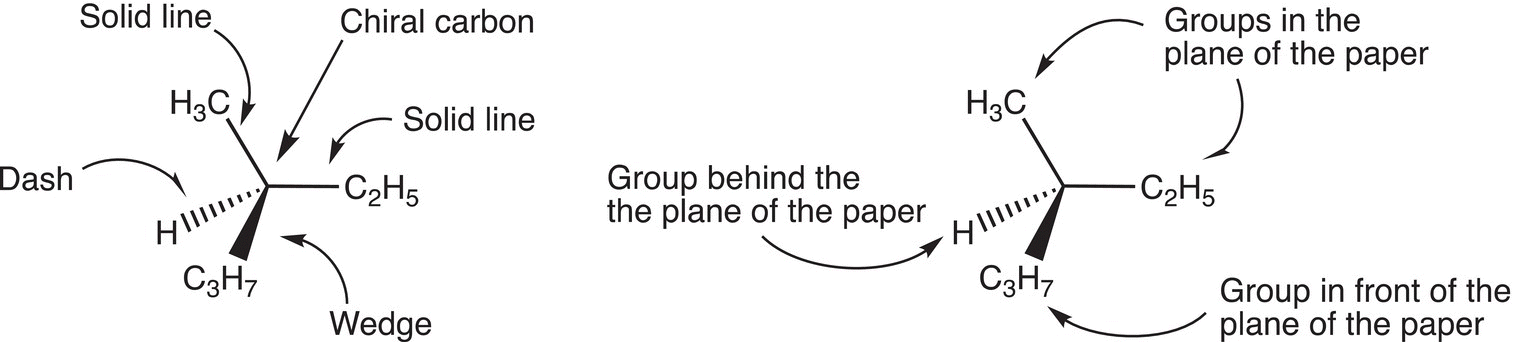
Figure 5.6 Illustration of the dashed-wedge representation for stereogenic molecules.
The dashed-wedge representation can also be used to show the stereochemistry of the 3-D arrangement of atoms or groups about a central carbon, and this type of representation is typically used to show the 3D stereochemistry about stereogenic carbons. Figure 5.6 gives examples in which dashed-wedge representations are used to show the stereochemistry about stereogenic carbons.
Problem 5.1
Molecules that are asymmetric or have a carbon that has four different groups are chiral. Utilize these two criteria to determine which of the following molecules are chiral?

The Fischer Projection is another representation that is typically used to represent a molecule in the three-dimensional space, and this type of representation is very useful to visualize the three-dimensional arrangements of the atoms in a molecule. Emil Fischer, a Nobel-prize winner, determined a method to visualize the configuration of an isomer that has four groups bonded to a central carbon. Compounds that are represented by the Fischer projection are viewed in such a way that the groups that are in front of the plane of the paper are located in a horizontal plane and the groups that are in a vertical plane are away from the viewer (in the back of the plane of the paper). The dashed-wedge representation can be used to illustrate the orientation of the Fischer projection and is demonstrated using 1-bromo-1-chloroethane shown in Figure 5.7.
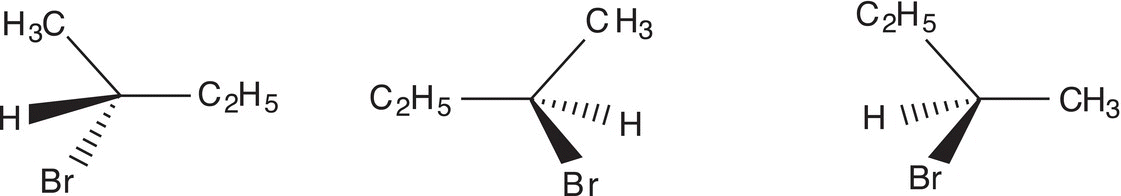
The Fischer projection and dashed-wedge representation for 2-bromobutane are given in Figure 5.8.
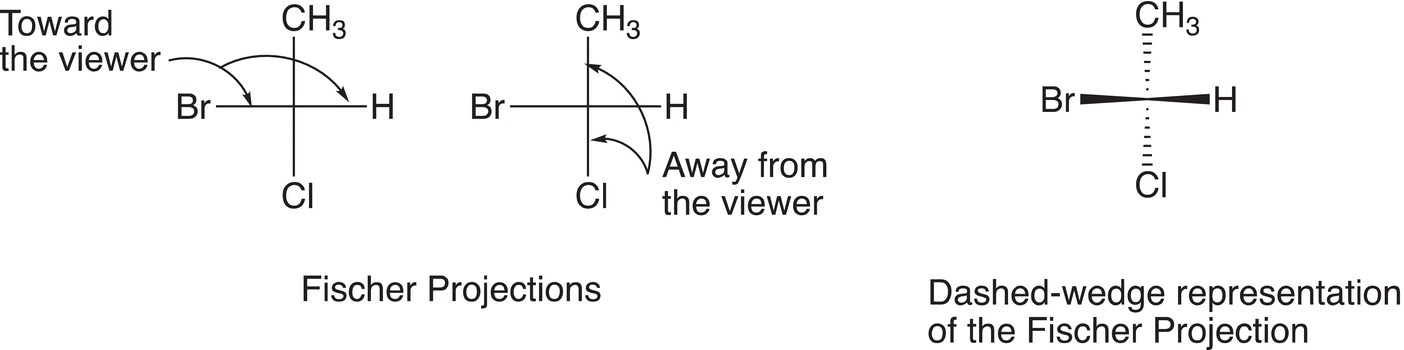
Figure 5.7 The Fischer projection of a specific isomer of 1-bromo-1-chloroethane.

Figure 5.8 Dashed/wedge and Fischer projection of 2-bromobutane.
Problem 5.2
1. Draw a Fischer projection of the following molecules.
1. 2-Chlorobutane (use C2 as the central carbon).
2. 2-Pentanol (use C2 as the central carbon).
3. 3-Hexanethiol (use C3 as the central carbon).
4. 3-Methyl-3-hexanol use (C3 as the central carbon).
2. Give the Fischer projection for each of the molecules shown below. Note that each molecule is represented by a specific dashed-wedge representation.