Organic Chemistry: Concepts and Applications - Headley Allan D. 2020
Bonding and Structure of Organic Compounds
1.4 Chemical Formulas
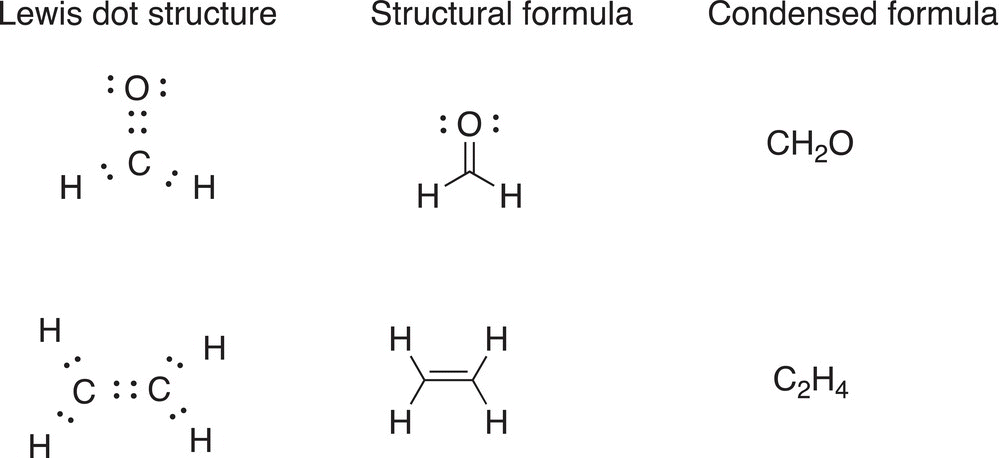
Condensed formulas show the types and fixed ratio of atoms that are contained in molecules. A number is used as a subscript to represent the same number of atoms in a molecule. The subscript is written to the right of the atoms in the condensed formula. Given condensed formulas for compounds, the Lewis dot structures give fairly good descriptions of the arrangements of the atoms, covalently bonded and nonbonded electrons in molecules and ions, but molecular formulas or structural formulas are used frequently to represent organic molecules. The Lewis dot structure of a molecule or ion can be transformed easily into structural formulas. For structural formulas, lines represent the bonding electrons of the Lewis dot structures, and dots are still used for the nonbonded electrons. Examples of both representations are shown below.
1.4.1 Line-Angle Representations of Molecules
Throughout the course, there is yet another representation of molecular structures that will be used and is known as the line-angle representation. In this representation, lines that intersect each other at an angle of 120° are used. Each intersection represents a carbon along with the appropriate number of hydrogens, but the hydrogens are not shown. For the line-angle representation, the start of a line represents a carbon with three hydrogens; at an intersection of two lines, there are two hydrogens; at an intersection of three lines, there is one hydrogen; and at the intersection of four lines, there are no hydrogens. Thus, for propane, shown below are the various representations, but as pointed out, the line-angle representation will be routinely used throughout this course.

Shown in Figures 1.15 and 1.16 are examples of a more detailed description of line-angle representations.
Shown below is another example in which different representations are used.
-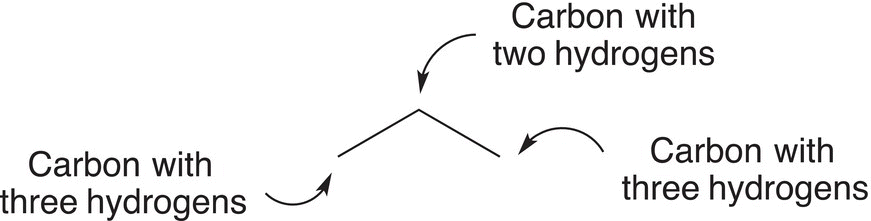
Figure 1.15 A detailed description of CH3CH2CH3 using a line-angle representation.
-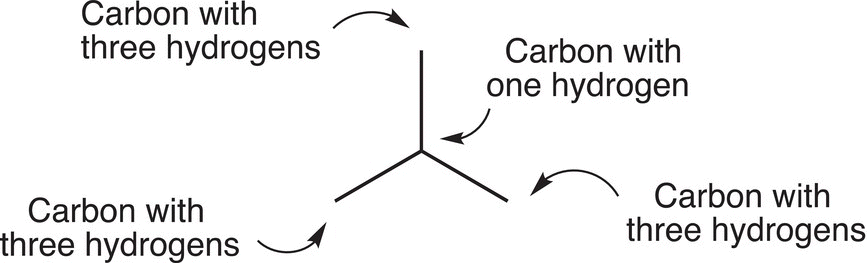
Figure 1.16 A detailed description of CH3CH(CH3)CH3 using a line-angle representation.
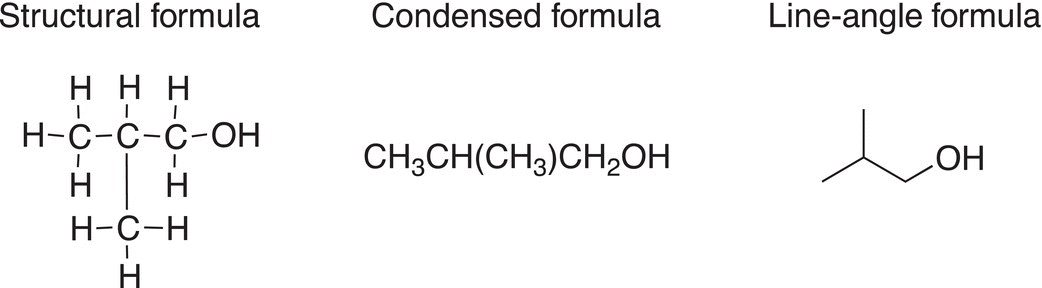
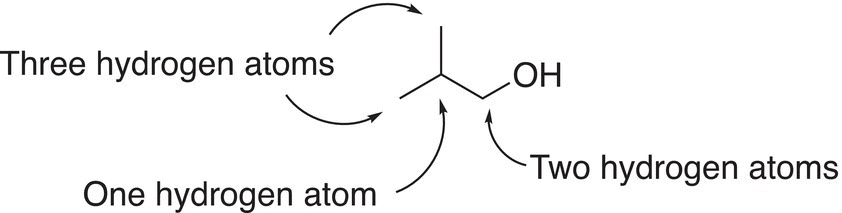
We will be using the line-angle representation mostly in this course and you should be very familiar with drawing this representation for different organic molecules. Shown below is a more detailed description of the line-angle representation for CH3CH(CH3)CH2COH.
Problem 1.13
Give the structural formulas (line-angle representation) for each of the following molecules.
1. CH3CH2CH2CH3
2. CH3C(CH3)2CH2CH3
3. CH3CH2CH(CH3)CH(CH3)CH3
Critical analytical thinkers should be able to not only convert condensed formulas to line angle but also the reverse, from line-angle to condensed. Problem 1.14 is designed to build on the ability of thinking in reverse.
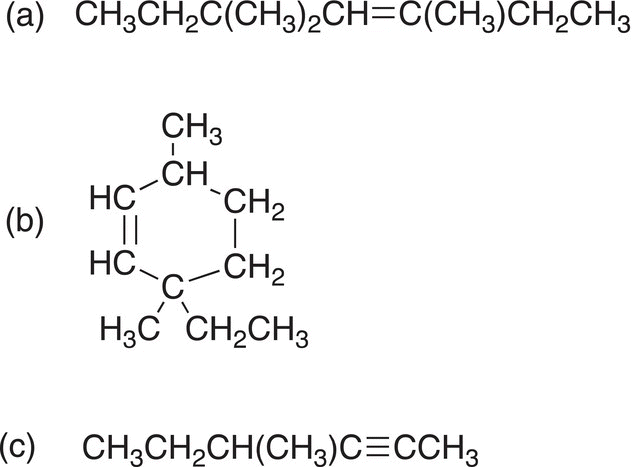
Problem 1.14
Give the condensed formulas for the following molecules.
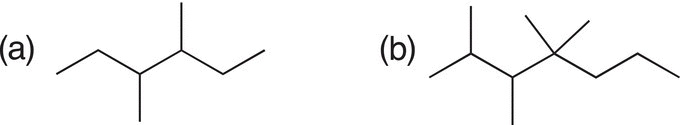
For molecules that have double bonds, the same line-angle representation is used, shown below are examples of acyclic and cyclic alkene molecules.
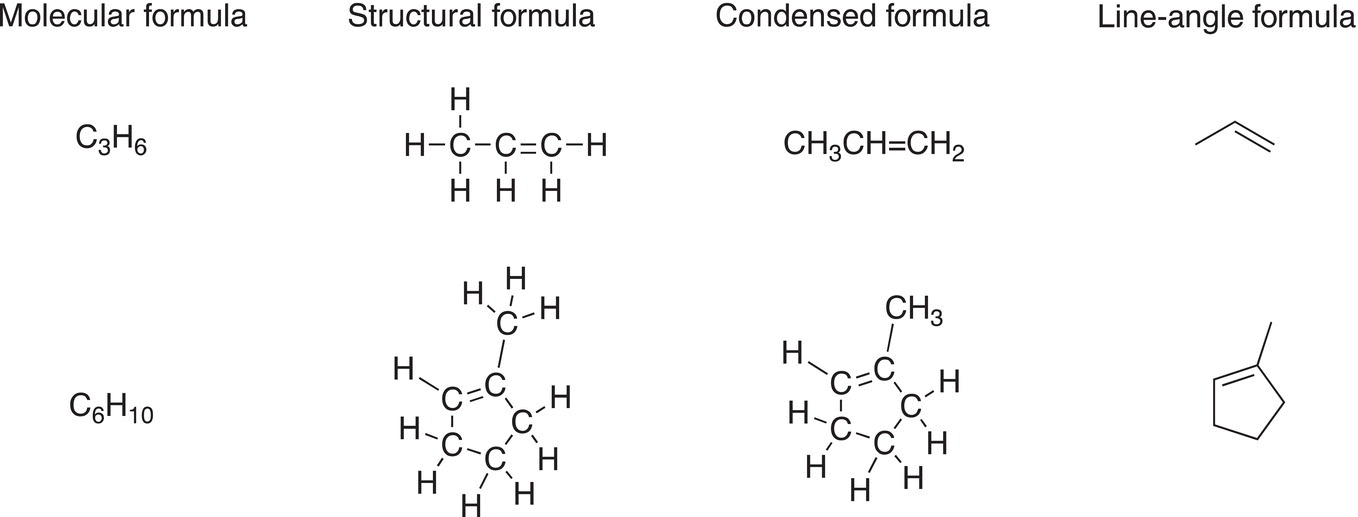
Note that the number of hydrogens is reduced by one for each carbon of the double bond, compared to the structures shown earlier. Also, the number of hydrogens is reduced by two for a cyclic compound, compared to acyclic compounds discussed earlier.
Owing to the linear geometry of the carbon atoms about triple bonds, the line-angle representation of a triple bond is drawn as linear as shown in the example below.
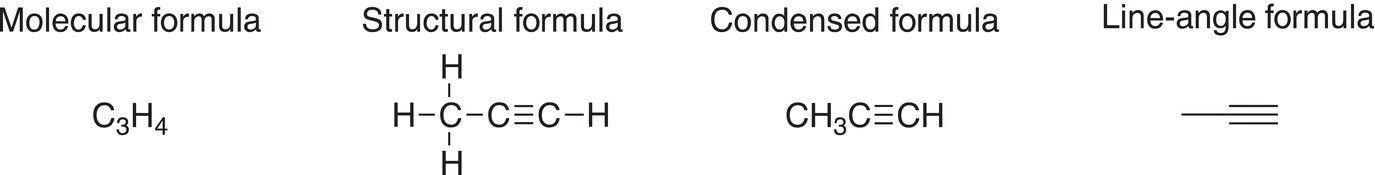
Problem 1.15
Give the structural formulas (line-angle representations) for each of the following molecules shown below.