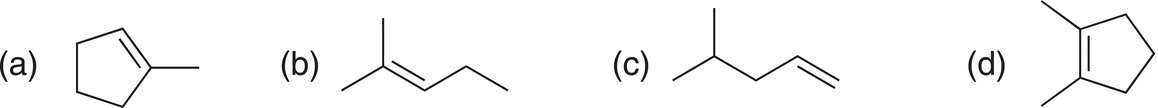Organic Chemistry: Concepts and Applications - Headley Allan D. 2020
Addition Reactions Involving Alkenes and Alkynes
8.3 Addition of Hydrogen Halide to Alkenes (Hydrohalogenation of Alkenes)
8.3.1 Addition Reactions to Symmetrical Alkenes
In this section, the knowledge gained in the previous section will be applied to real molecules to not only predict the organic products, but the major organic product. The first reaction that will be examined is the addition of hydrochloric acid (H-Cl) to cyclohexene as shown in Reaction (8-8).
(8-8)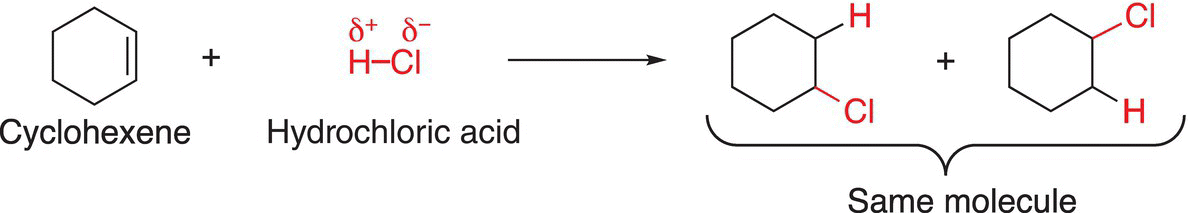
Recall that the H-Cl bond is a polar covalent bond and due to chlorine being more electronegative, compared to hydrogen, the bonding electrons are closer to the chlorine, compared to the hydrogen. Hence, the hydrogen becomes the electrophile and the counter chloride anion is a nucleophile. Thus, the first step of the reaction mechanism will involve the addition of the electrophilic H+ to the carbon—carbon double bond of cyclohexene as shown in the elementary step of Reaction (8-9) to form a carbocation.
(8-9)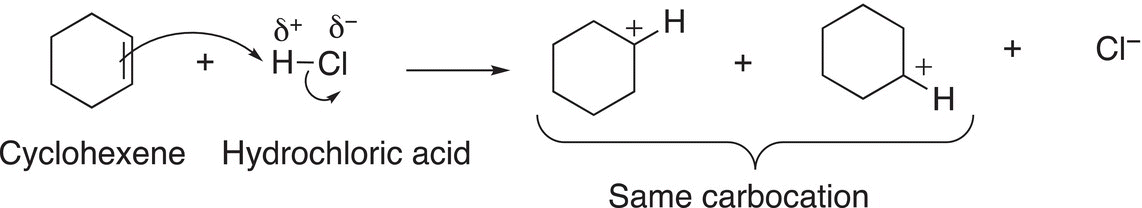
Note that since the alkene is symmetrical, the same carbocation results after the addition of the proton to either carbon of the carbon—carbon double bond. As a result, only one organic product will be formed from the second step in the mechanism, where the nucleophilic chloride anion adds to the carbocation, as shown in Reactions (8-10) and (8-11).
(8-10)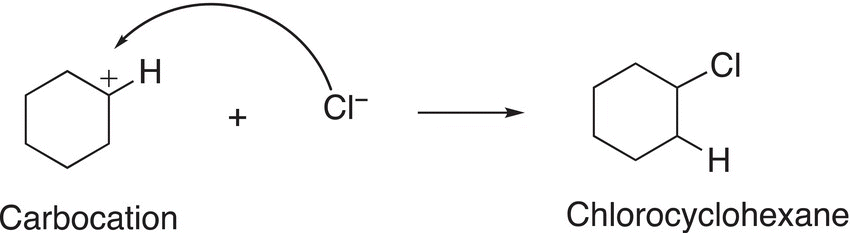
(8-11)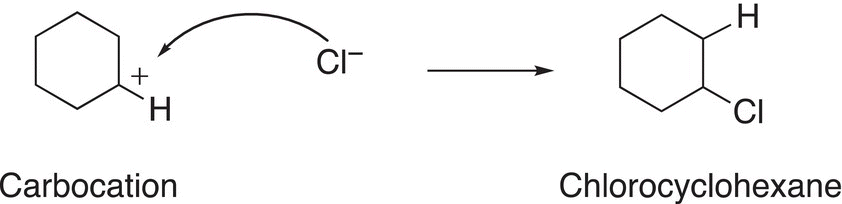
Problem 8.1
i. Give the products for the reactions shown below: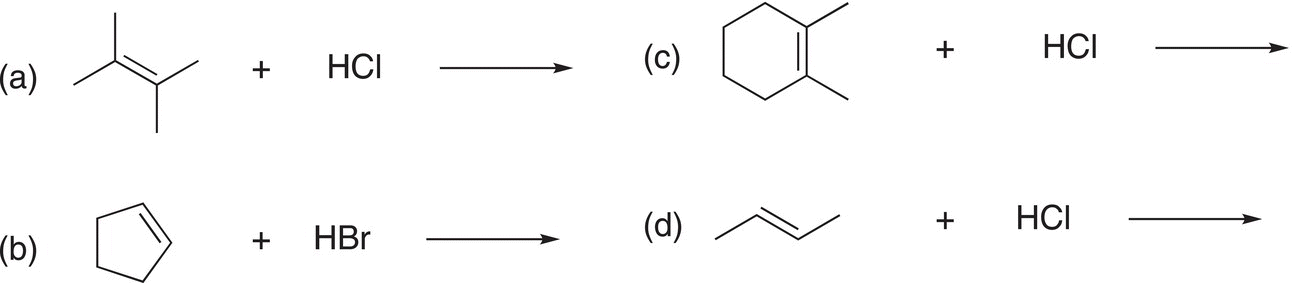
ii. Are there two different or one addition product that results from each of the above reactions? Explain your answer.
8.3.2 Addition Reactions to Unsymmetrical Alkenes
Based on the information presented in the previous section, if the alkene is symmetrical, only one product results from the addition of HCl or similar molecules. On the other hand, if the alkene is not symmetrical, that is there are different groups bonded to the carbons of the double bond, then two possible carbocations result, as shown in Reaction (8-4). The addition of the nucleophilic anion to these carbocations results in two different electrophilic addition products, as shown in Reaction (8-12).
(8-12)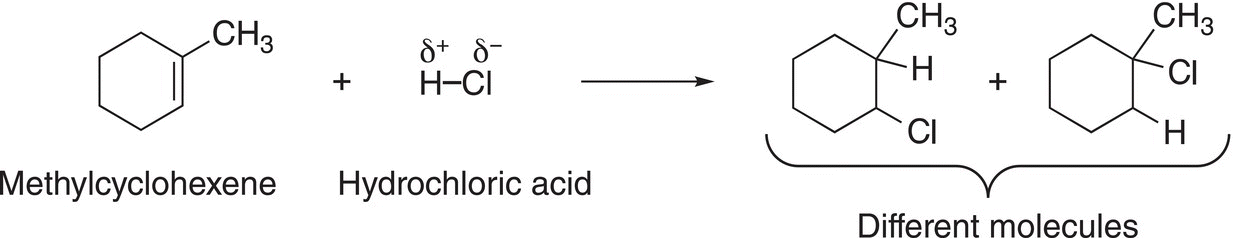
One of these products is the major organic product and the method to determine which is the major product will be discussed in the next section.
Problem 8.2
Give the two possible products for the reactions shown below:
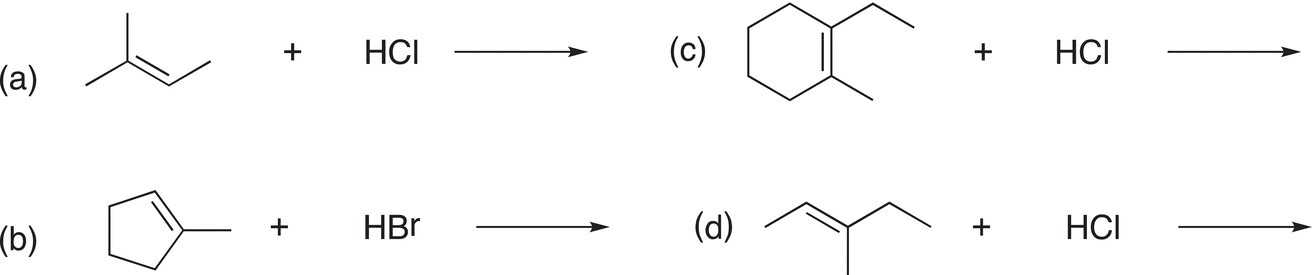
8.3.3 Predicting the Major Addition Product
For the reaction involving methylcyclohexene and hydrochloric acid (Reaction 8-12), one of the products shown is the major product and the other is the minor product. In order to be able to predict the major organic product, a close examination of the reaction mechanism must be carried out. It should be remembered that in the first step of the mechanism, carbocations are the intermediates that result from the initial addition of the electrophile (H+) to the nucleophilic double bond (Reactions 8-4 and 8-9). For this reaction however, one of these carbocations is a more stable carbocation than the other and will be formed in greater abundance compared to the less stable carbocation. Thus, a systematic method must be established to determine the relative stabilities of carbocations.
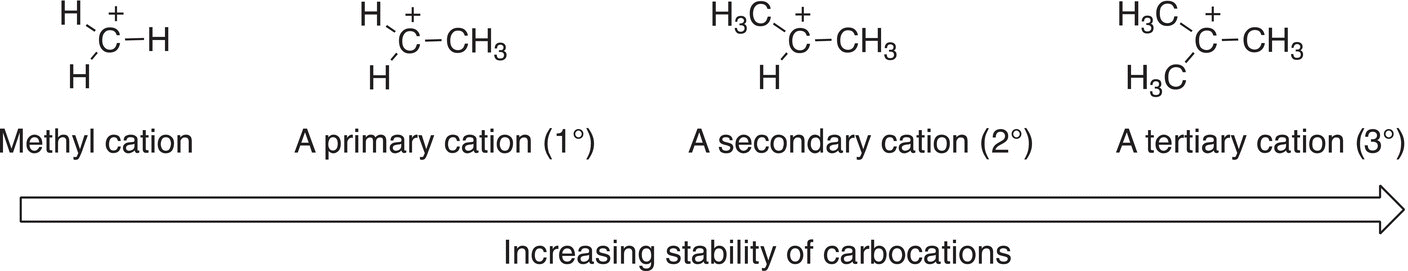
A carbocation that has three alkyl groups bonded to the carbon of the carbocation is more stable than one that has two alkyl groups bonded to the carbon of the carbocation. A carbocation that has three alkyl groups bonded to the carbon of the carbocation is called a tertiary carbocation (3°), whereas a carbocation that has two alkyl groups bonded to the carbon of the carbocation is called a secondary carbocation (2°). Alkyl groups, which are electron rich, can donate electrons to help stabilize the positive charge of a carbocation. Thus, a tertiary carbocation, which has three alkyl groups, is more stable than a secondary carbocation, which has two alkyl groups. Based on this trend, it can be readily concluded that a carbocation that has only one alkyl group bonded to the carbon of the carbocation is called a primary (1°) and is not as stable as a secondary carbocation or a tertiary carbocation. The methyl carbocation is therefore the least stable carbocation. Figure 8.2 gives illustrations of the structure of carbocations and how adjacent alkyl groups can stabilize different types of carbocations.
Thus, the order of the stability of different carbocations is as shown below:
A different representation of the order of the stability of carbocations is shown below.
Tertiary (3°) > Secondary (2°) > Primary (1°) > Methyl (CH3)
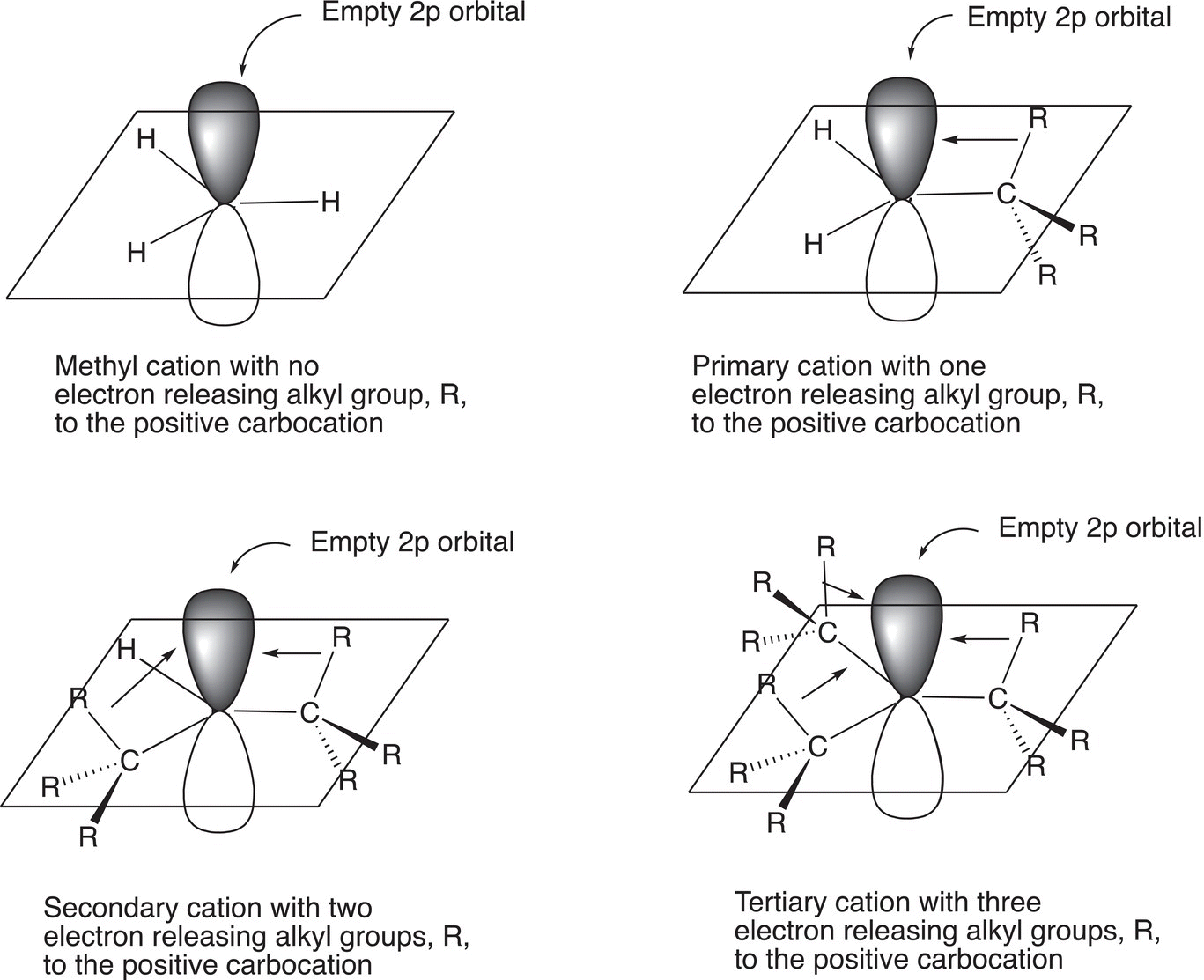
Figure 8.2 Relative stabilities of different types of carbocations.
The addition of an electrophile, such as the proton, to the carbon—carbon double bond of methylcyclohexene results in two possible carbocations, which are shown in Reaction (8-13).
(8-13)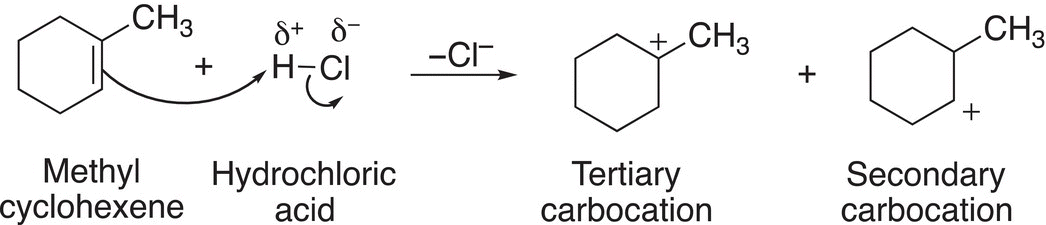
In the final step of the mechanism, the nucleophilic chloride anion adds to the carbocation to form the products, but since the tertiary carbocation is more stable and formed in greater abundance, compared to the secondary carbocation, the tertiary carbocation will lead to the major organic product, as shown in Reactions (8-14) and (8-15).
(8-14)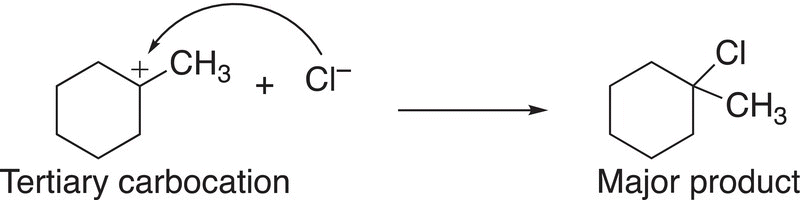
(8-15)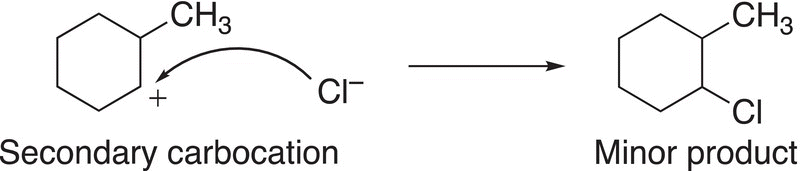
The overall reaction is shown in Reaction (8-16), and the Figure 8.3 shows the energy profile for the two steps in the addition reaction of hydrochloric acid to methylcyclohexene to give two intermediates, a more stable tertiary carbocation (lower in energy), compared to the secondary carbocation, which is higher in energy.
(8-16)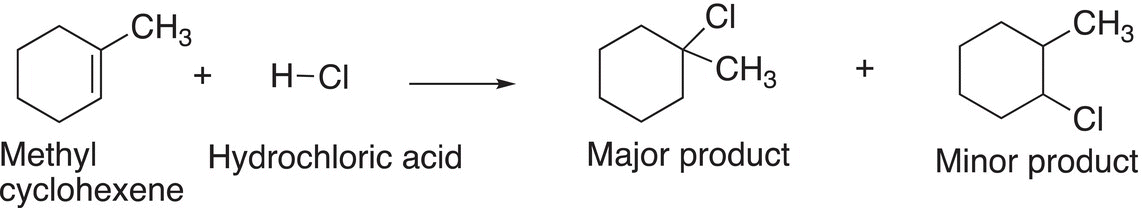
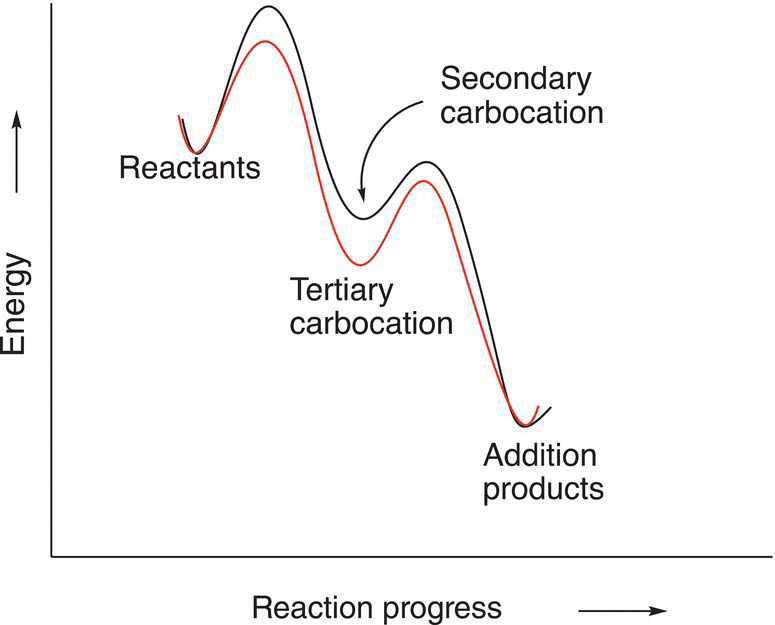
Figure 8.3 Energy profile for the addition reaction of methylcyclohexene with hydrochloric acid.
This analysis of determining the relative stability of the intermediate carbocation in order to predict the major addition product can be applied to similar addition reactions involving different alkenes. The addition of hydrochloric acid to propene results in two products as shown in Reaction (8-17). During the course of the reaction, a primary and a secondary carbocation are formed, but the major product results via the secondary and more stable carbocation.
(8-17)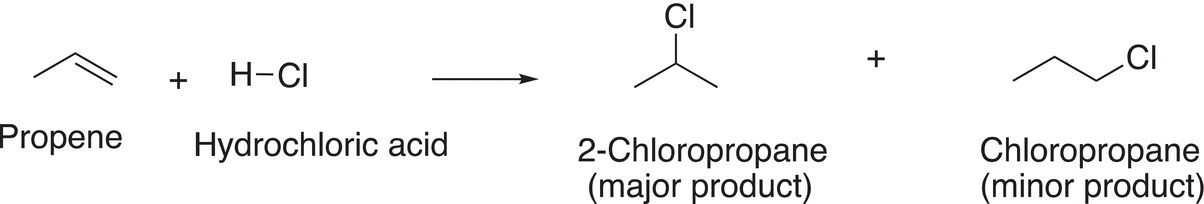
The first step of the mechanism involves the addition of the electrophilic proton of the hydrochloric acid to the nucleophilic double bond of the alkene to form two possible carbocations as shown in Reaction (8-18).
(8-18)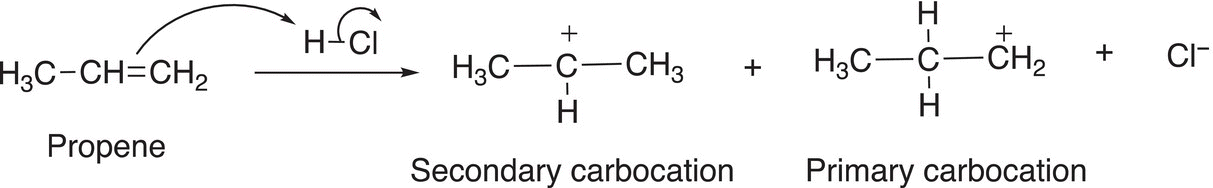
As pointed out earlier, a primary carbocation is one that has only one alkyl group that is bonded to the carbon of the carbocation. As a result, it is less stable than either a secondary or a tertiary carbocation. Owing to the greater stability of the secondary carbocation, compared to the primary carbocation, it will be in greater abundance and hence lead to the major organic product after the addition to the nucleophilic chloride anion, as shown in Reaction (8-19) with the primary carbocation leading to the minor product, as shown in Reaction (8-20).
(8-19)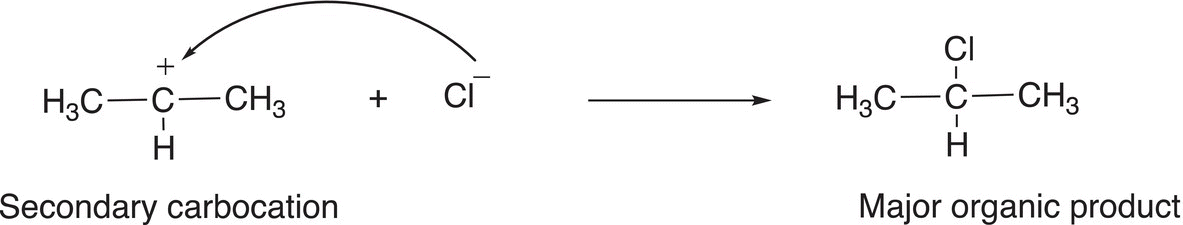
(8-20)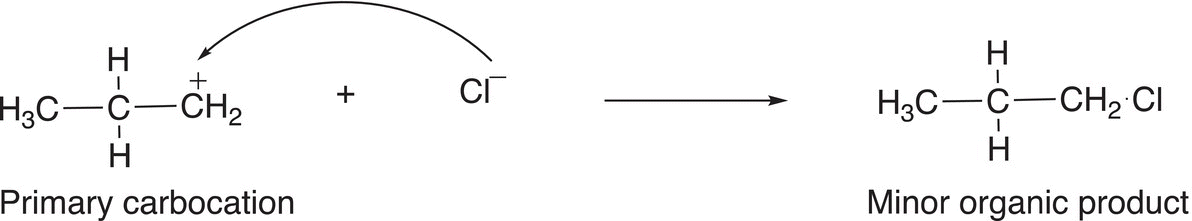
8.3.4 Predicting the Stereochemistry of Addition Reaction Products
Since the carbon of carbocations is sp2 hybridized, there is a vacant p orbital, which is orthogonal to the plane of the sigma (σ) bonds. As a result, the nucleophile can attack from either side of the flat carbocation as illustrated in Figure 8.4.
Thus, there will be an equal mixture of enantiomers making the product racemic as shown in Reaction (8-21) if a stereogenic carbon is generated in the product.
(8-21)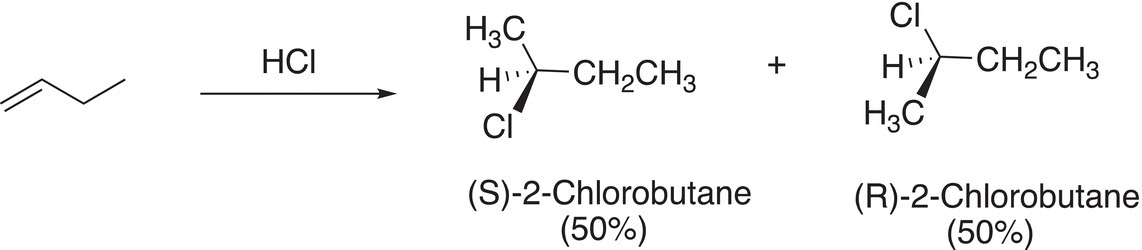
8.3.5 Predicting the Major Addition Product — Markovnikov Rule
Markovnikov, a Russian Chemist, was one of the first scientists to predict the major organic product for addition reactions involving the addition of H+Nu− across a double bond. He predicted that the major product of these addition reactions is the one in which H+ adds to the carbon of the alkene double bond that has the most hydrogens. This observation is now known as Markovnikov's Rule. For the reaction shown below, the hydrogen of HCl adds to the carbon with two hydrogens instead of the carbon with only one hydrogen to give the major product.
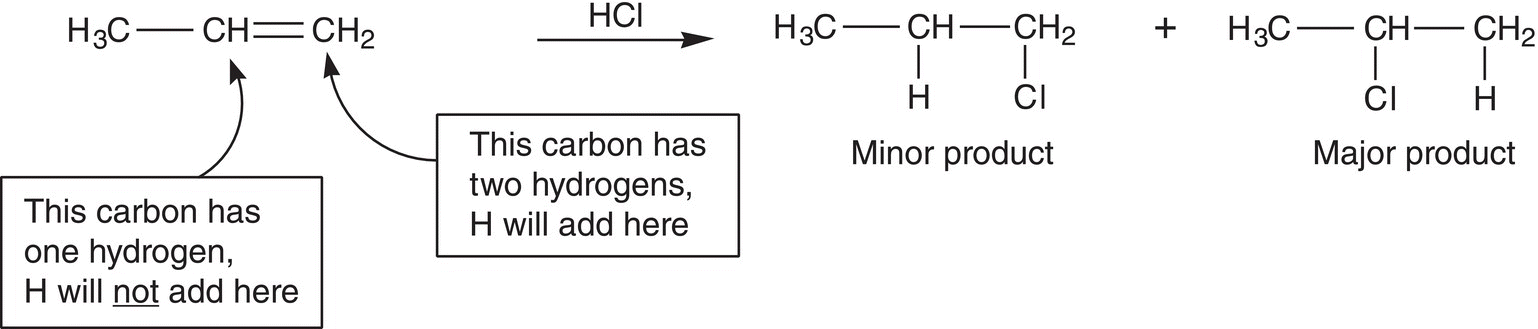
For electrophilic addition reactions, the major product results from the addition of the electrophile to the carbon that has the most hydrogens and the nucleophile adds to the carbon that has the least hydrogens. As a result, these reactions are known regiospecific reactions since the electrophile and the nucleophile add to specific regions of the reactant molecule.
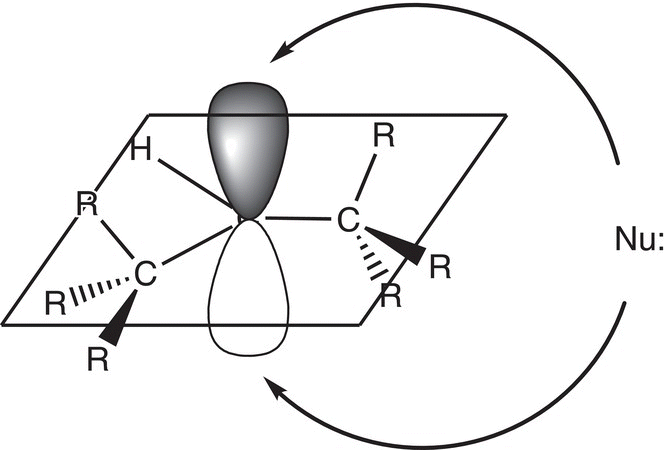
Figure 8.4 Flat carbocation showing equal probability of attack of nucleophile for either side.
Problem 8.3
Using Markovnikov's rule, predict the major product that results from the addition of HCl to the following alkenes.
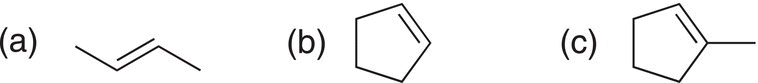
8.3.6 Unexpected Hydrohalogenation Products
For a large percentage of the reactions that will be encountered in organic chemistry, there are sometimes products that are obtained that are unexpected. To better understand the formation of unexpected products, it is important to know mechanistic possibilities for the reactions. For electrophilic addition reactions, often times, the occurrence of unexpected organic products is due to the rearrangement of carbocation intermediates to form more stable carbocations. Consider Reaction (8-22).
(8-22)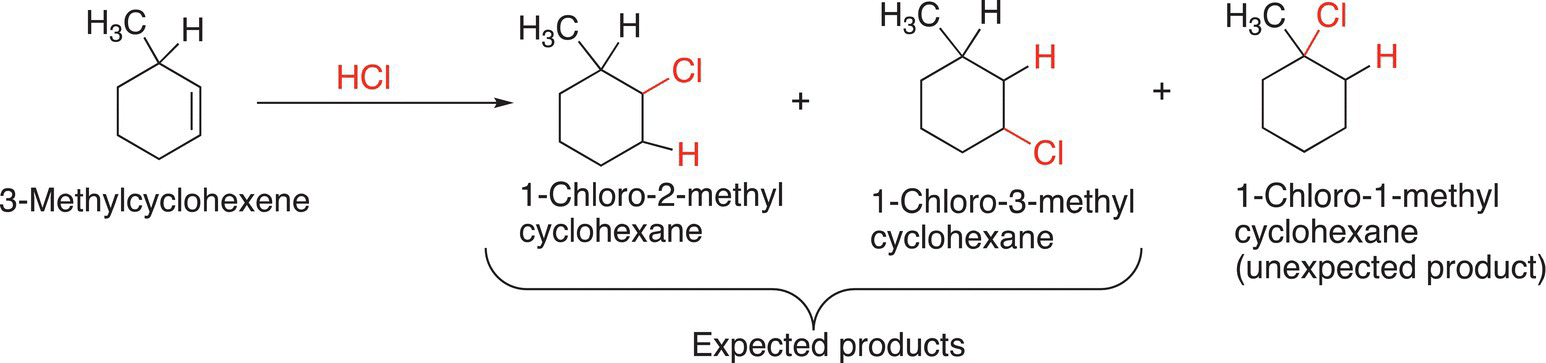
The first step of the mechanism for this reaction involves the addition of the electrophilic proton to the nucleophilic alkene, as shown in Reaction (8-23).
(8-23)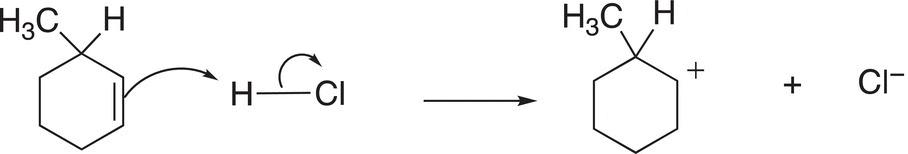
The secondary carbocation that is formed can be converted to a tertiary carbocation by a hydride shift as shown in Reaction (8-24), which eventually leads to the unexpected organic product.
(8-24)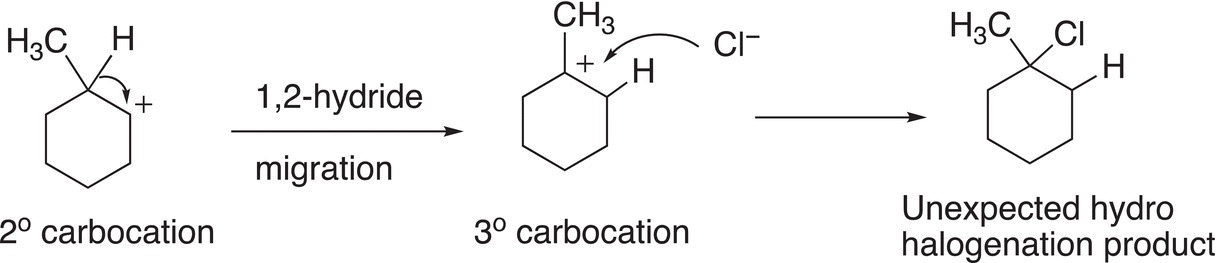
For the addition reaction of hydrochloric acid to 3,3-dimethylcyclohexene, an unexpected product is obtained, as shown in Reaction (8-25).
(8-25)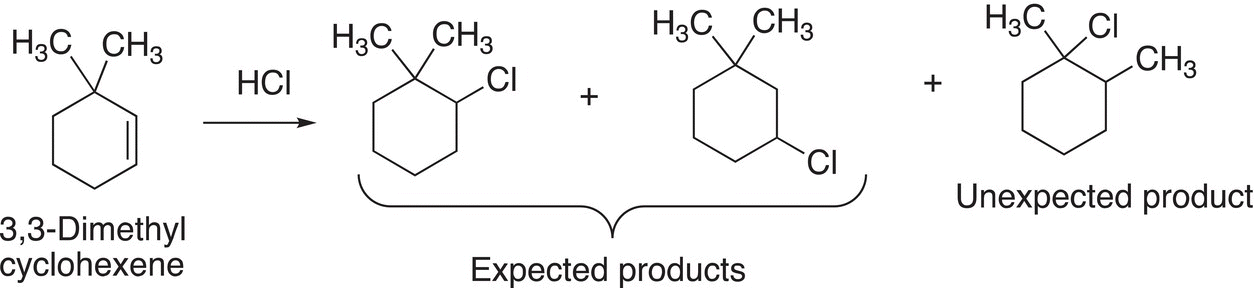
The first step of the mechanism for this reaction is similar to Reaction (8-23) and is shown in Reaction (8-26).
(8-26)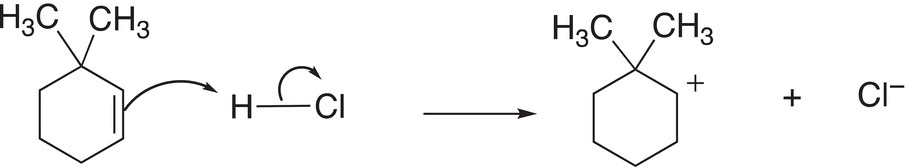
In this case, the secondary carbocation that is formed can rearrange to form a tertiary carbocation, but in this case, a methyl group with its bonding electrons (methide anion) migrates as shown in Reaction (8-27) to form a tertiary carbocation, which eventually leads to the unexpected organic product.
(8-27)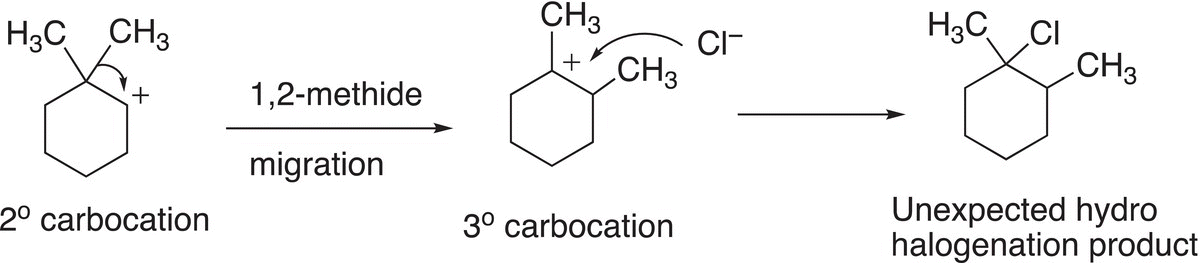
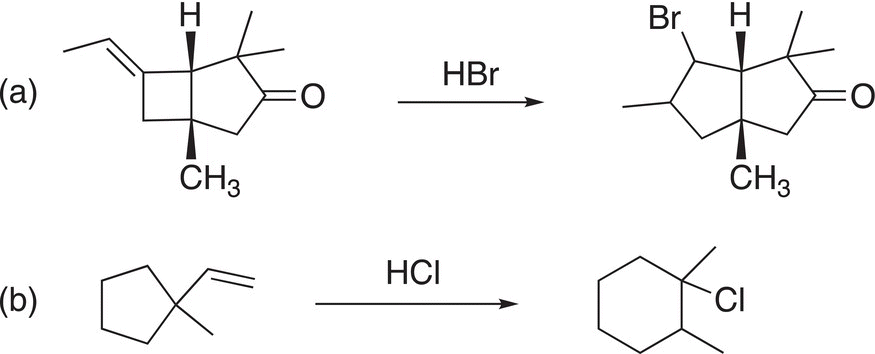
For some carbocations, the methyl group is not the only group that can migrate, but sometimes an adjacent sigma bond in order to create a more stable cation as needed to explain the unexpected products shown in Problem 8.4.
Problem 8.4
Using arrows to represent electron movement, provide a reasonable mechanism to explain the following reactions.
8.3.7 Anti-Markovnikov Addition to Alkenes
A challenge that chemists are often faced with is to obtain the anti-Markovnikov product as the major organic product. That is, the addition of the electrophile and nucleophile in an anti-Markovnikov manner, as shown in Reaction (8-28).
(8-28)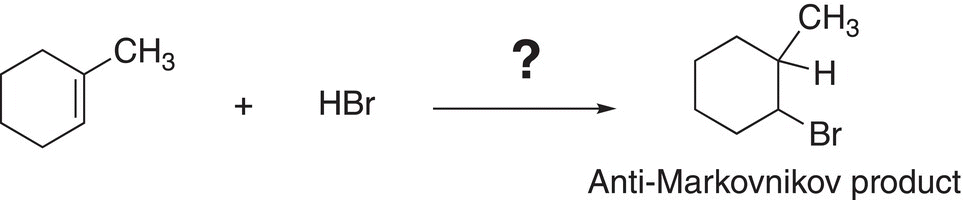
In order to determine if Reaction (8-28) is possible, the mechanism by which it would most likely occur will have to be examined. In order to fully understand how best to take advantage of the chemistry that will make this occur, possible mechanisms by which reactions occur must be examined. It is obvious that just adding HBr will not work since the major product would be the Markovnikov product owing to the most stable carbocation intermediate formed during the course of the reaction. As a result, a different approach must be used and the mechanism for this reaction would have to be different from the regular mechanism discussed earlier. It is known that H-Br, in the presence of peroxide and heat, generates the bromine radical and the use of radical intermediates, instead of carbocation intermediates, would produce a different mechanism for the addition of H-Br to a carbon—carbon double bond.
Radicals are very important intermediates in chemistry; radicals are reactive intermediates and are typically generated by the homolytic cleavage of a sigma bond. One of the most common ways of generating free radicals is the homolytic breakage of the oxygen—oxygen bond of peroxides. In the presence of energy, such as heat or light, an oxygen—oxygen bond will break homolytically to produce radicals as shown in Reaction (8-29).
(8-29)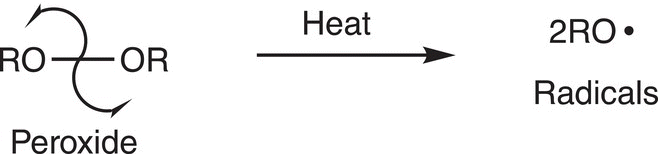
Examples of common peroxides that are used in organic chemistry to generate radicals are shown in Reactions (8-30) and (8-31).
(8-30)
(8-31)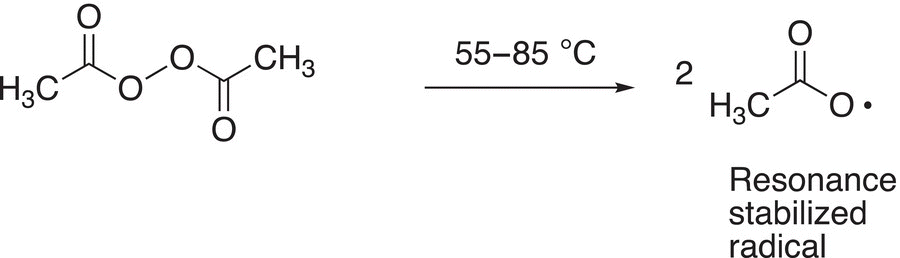
Note that the radical generated in Reaction (8-30) requires a fairly high temperature, whereas less energy is required for the generation of the more resonance-stabilized radical shown in Reaction (8-31). Another type of compound that is often used to generate radicals is azoalkane, such as the one shown in Reaction (8-32).
(8-32)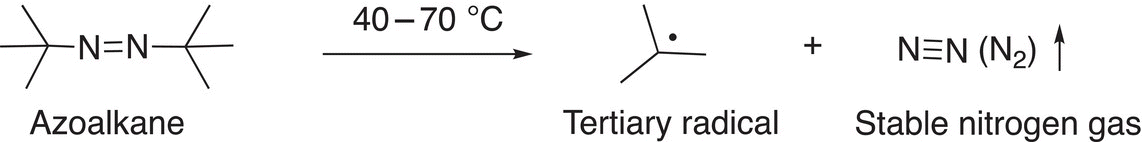
The energy requirement for the generation of the radical of Reaction (8-32) is the least of the three reactions. Since this reaction liberates nitrogen as a gas, the reaction is entropy favored. Reactions that generate radicals as those given above are typically referred to as radical initiation reactions or steps.
Since radicals are reactive intermediates, they provide an alternative mechanism by which H-Br can add in a different manner across a carbon—carbon double bond. The bromine radical can be generated from the elementary reaction as shown in Reaction (8-33) in which the radical abstracts a hydrogen radical from HBr to generate another radical. This step is also known as radical propagation step since radicals are produced in a reaction with another radical.
(8-33)![]()
Once bromine radicals are produced, even though it is fairly stable owing to its size, it will add to an unsymmetrical alkene to generate two carbon radicals as shown in Reactions (8-34) and (8-35).
(8-34)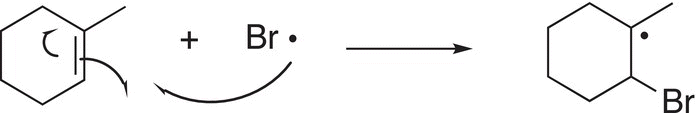
(8-35)
Since there are two carbon radicals that are generated, the more stable will be formed in greater abundance and lead to the major product. Carbon radicals are similar to carbocations except that carbon radicals have one electron in the adjacent unhybridized p orbital, compared to carbocations, which have no electrons in the p orbital. As a result, a similar analysis as that carried out to determine the stability of carbocations can be used to determine the stability of radicals.
A carbon radical that has three alkyl groups bonded to the carbon of the radical is more stable than the one that has two groups bonded to the carbon of the radical. A radical that has three alkyl groups bonded to the carbon of the radical is called a tertiary radical (3°), whereas a radical that has two alkyl groups bonded to the carbon of the radical is called a secondary radical (2°). Alkyl groups, which are electron rich, can donate electrons to help stabilize the single electron of a radical. Thus, a tertiary radical, that has three alkyl groups bonded to the carbon of the radical, is more stable than a secondary radical, which has two alkyl groups bonded to the radical carbon. A radical that has only one alkyl group bonded to the carbon of the radical is called a primary (1°) and is not as stable as the secondary or the tertiary radicals. Figure 8.5 gives an illustration of the stability of radicals. The methyl radical is therefore the least stable radical and a tertiary radical is the most stable.
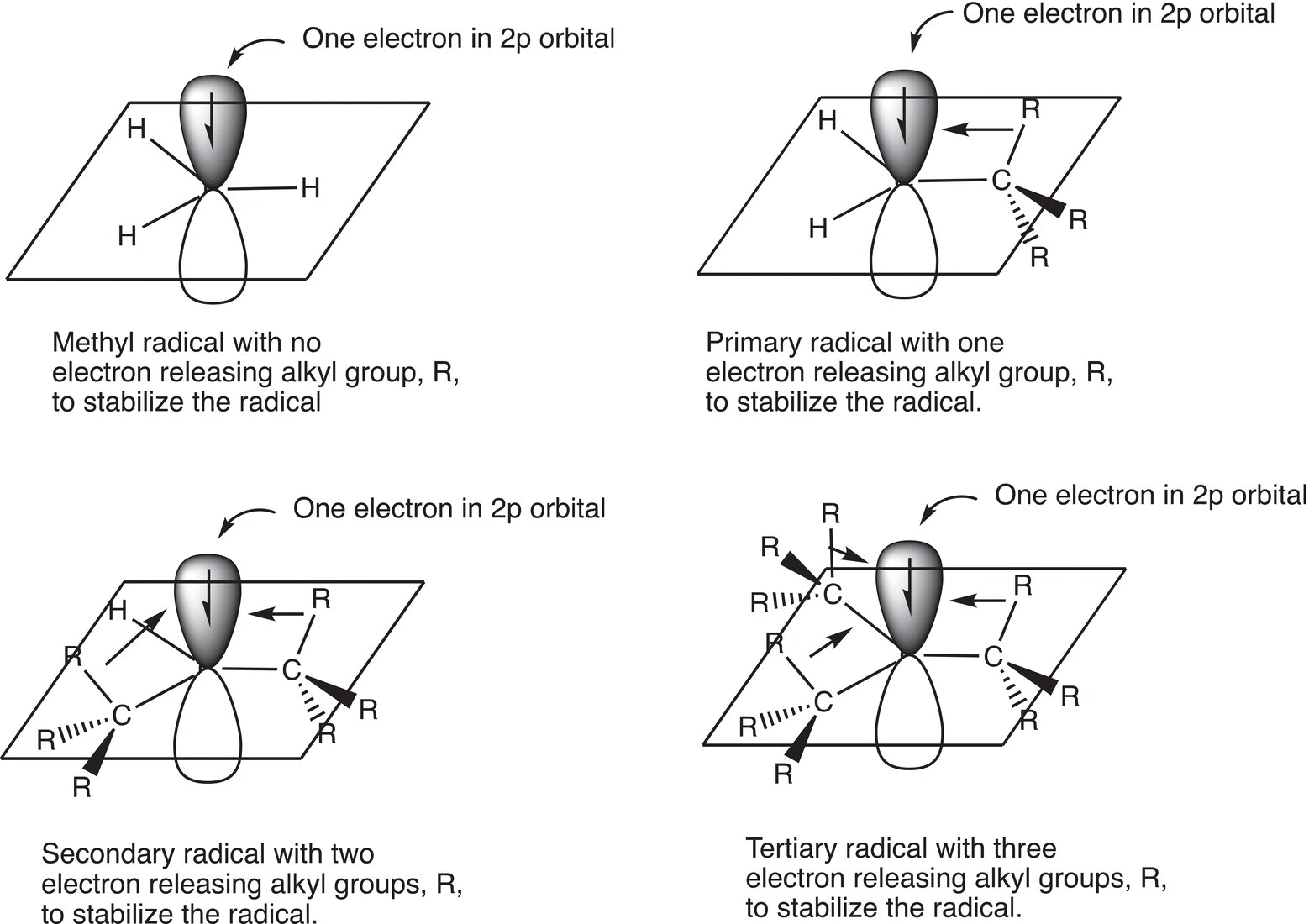
Figure 8.5 Relative stabilities of different types of radicals.
Thus, the order of stability of different radicals is as shown below:
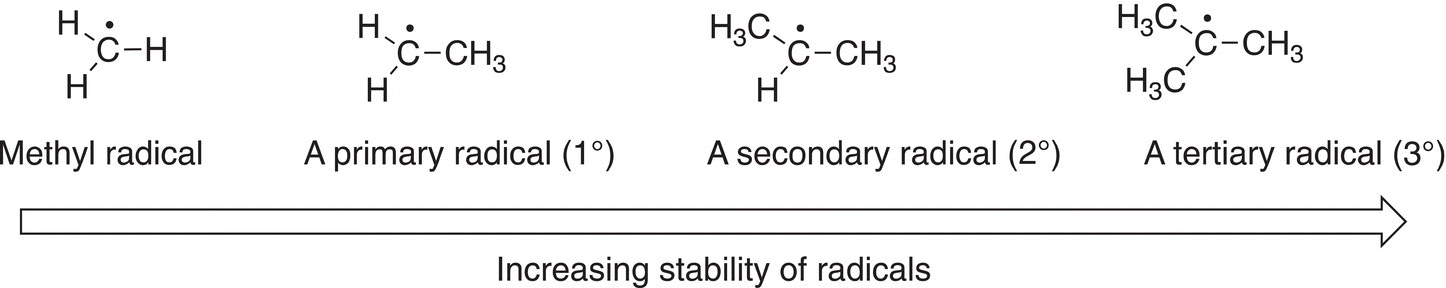
A different representation of the order of the stability of radicals is shown below.
Tertiary (3°) > Secondary (2°) > Primary (1°) > Methyl (CH3)
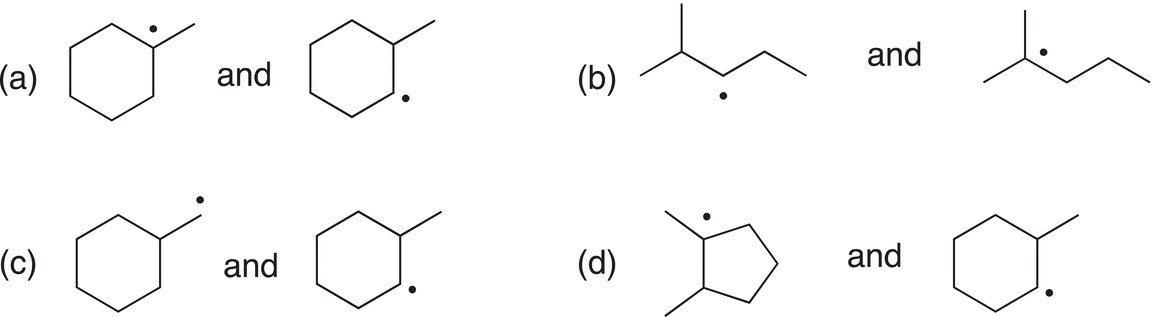
Problem 8.5
Consider each of the following pairs of radicals, then determine which radical is the most stable.
Based on the stability trend for radicals, the radicals produced in Reactions (8-34) and (8-35) can be summarized as shown in Reaction (8-36), one radical is tertiary and more stable and the other radical is a secondary and less stable.
(8-36)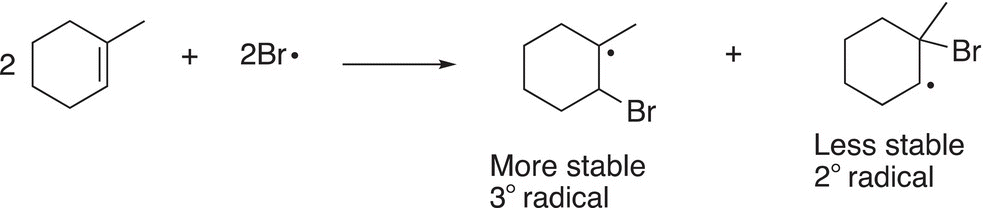
Typically, radicals are not as prone to rearrangement as carbocations and there are no rearranged unexpected products for these reactions. The products will be obtained from these two radicals, but the major product will result from the more stable radical intermediate as shown in Reactions (8-37) and (8-38).
(8-37)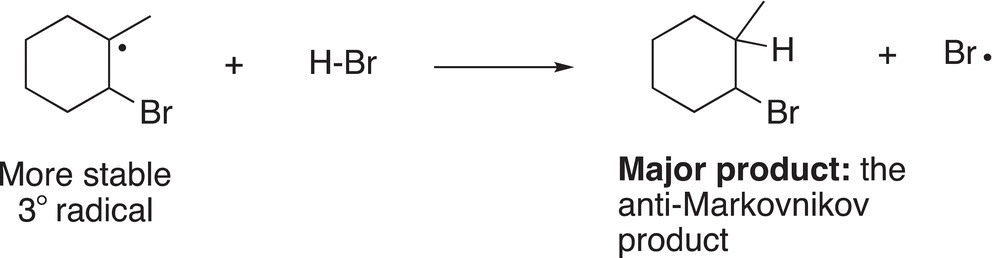
(8-38)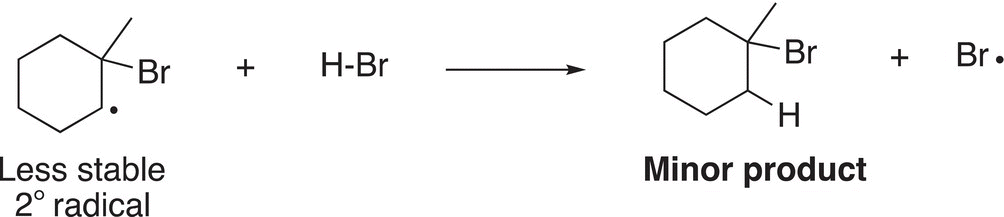
For these reactions, the radical reacts with H-Br to produce the organic products and another bromine radical. Note that the major product will be the one produced in Reaction (8-37) since the tertiary radical is more stable than the secondary radical.
In a final step where radicals react to form a neutral molecule, also known as the termination steps, bromine radicals react with another radical to form either Br2 or ROBr as shown in Reactions (8-39) and (8-40).
(8-39)![]()
(8-40)![]()
There are other possibilities for the termination of radicals involving coupling of the carbon radicals, but the reaction conditions can be controlled to produce the desired product. Thus, the overall reaction for the hydrobromination of methylcyclohexene in the presence of peroxide is given in Reaction (8-41). Note that it is a regiospecific reaction since for the major product, the bromine adds to the carbon with the most hydrogens, and the hydrogen adds to the carbon with the least number of hydrogens.
(8-41)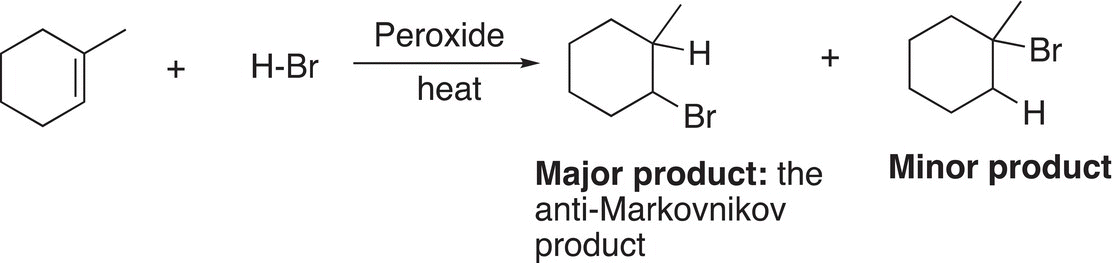
Gaining different outcomes of these addition reactions (Markovnikov and anti-Markovnikov reactions) by a slight change in reaction conditions demonstrates the importance of understanding the mechanisms of reactions. Chemists can manipulate reaction conditions to get a high percentage of a major organic product or to get a totally different organic product. Reaction (8-42) gives a summary of this concept that slight changes in reaction conditions can lead to different products.
(8-42)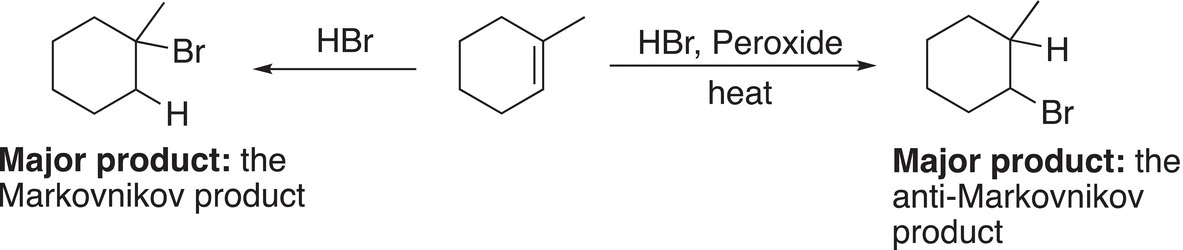
Problem 8.6
Give the major product that results from the addition of HBr to the following alkenes in the presence of peroxides and heat.