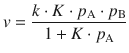Physical Chemistry Essentials - Hofmann A. 2018
Catalysis
7.2 Heterogeneous Catalysis
We have established earlier that catalysts accelerate reactions, but do not undergo a net chemical change themselves. Catalysis is thus achieved by lowering the activation energy of a reaction, and by providing an alternative path to circumvent the slow, rate-determining step of the non-catalysed reaction.
A heterogeneous catalyst, exists in a different phase than the reaction mixture; its catalytic activity builds on the capability to bring the reactants into very close spatial vicinity. A typical example is the olefin hydrogenation reaction catalysed by metals such as palladium, platinum or nickel.
If we consider a heterogeneous catalytic process involving solids, we need to conceptualise the attachment of particles to the surface of the solid ( adsorption), as well as the reverse process ( desorption). The substance that attaches to the surface is called the adsorbate; the underlying material is called the adsorbent or substrate.
7.2.1 Solid Surfaces
While the surface of solids appears flat from a macroscopic perspective, this perception is a matter of resolution. Indeed, at sub-microscopic level, surfaces of solids are not flat but show various features and defects. At atomic detail, the flat surface looks like a layer of oranges in the grocery store. Importantly, there may be higher order in solids whereby the constituting atoms, ions or molecules are arranged in particular repeating structures. Such structures are called a crystalline lattice and the solid is then called a crystal.
The terrace step kink (also known as terrace ledge kink) model describes the surface formation and transformations of crystals, whereby the energy of a particular atom on a surface depends on the number of bonds that atom has with neighbouring atoms (Stranski 1928). As the term ’terrace step kink model’ implies, the typical features on surfaces are terraces, steps and kinks (see Fig. 7.7).
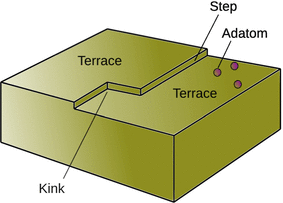
Fig. 7.7
Schematic illustration of the terrace step kink model of solid surfaces
When an atom arrives at a terrace, it is called an adatom and may bounces across the surface depending on the inter-molecular potential—this process is called accommodation (see Physisorption in the following section). If it comes to lie in a kink (kink atom) or at a step (step atom), it can interact with more than one surface atom, and the interaction may be strong enough to trap it. When ions deposit from solution, the loss of solvation energy is offset by strong Coulomb forces (i.e. the electrostatic forces) between the arriving ions and ions on the surface.
Macroscopically, many crystals are recognised by their shape which is constituted by flat faces with sharp angles. A distinct shape is not a necessary criterion for a solid to be a crystal, but it is a frequently observed property. How quickly a surface (or face) growths on a crystal, depends on the particular plane. As illustrated in Fig. 7.8, the slowest growing faces dominate the overall shape of the crystal.
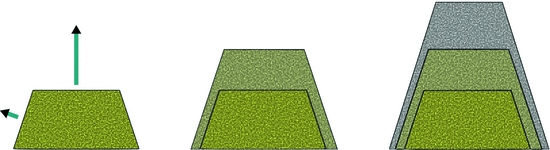
Fig. 7.8
Different faces of a crystal grow at different speeds, indicated by the length of the turquoise arrows. Evidently, the slowest growing faces dominate the overall shape and macroscopic appearance of a crystal
7.2.2 Adsorption
When particles adsorb to surfaces, a fundamental observable is the fractional coverage of the available surface area. As occupancy of a surface area comes about by particles occupying individual sites, the fractional coverage Γ can be defined as per
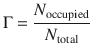
(7.10)
Here, N occupied is the number of occupied adsorption sites and N total is the number of total adsorption sites on the surface. Another frequently used definition expresses the fractional coverage Γ in terms of the volume of adsorbate (V) and the volume of adsorbate required for total coverage (V ∞):
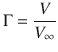
(7.11)
The rate of adsorption is the rate of change of surface coverage (dΓ) over time (dt) and can be determined using the observables of Eqs. 7.10 or 7.11:
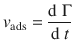
(7.12)
Two types of adsorption are typically distinguished, based on the types of forces involved in the interaction between adsorbate and adsorbent: physisorption and chemisorption. The problem of distinguishing between the two types is akin to that of distinguishing between chemical and physical interactions in general.
Physisorption
If molecules or atoms attach to a surface exclusively due to van der Waals interactions, this adsorption process is called physisorption. This type of adsorption is for example observed in dispersions and arises due to dipolar interactions. van der Waals interactions are long-range interactions of rather low energy. The rather small enthalpies of physisorption (~ −20 kJ mol−1) are not sufficient to break bonds, so the adsorbate retains its identity on the surface; geometric distortions may be possible, though. Despite being rather weak interactions, there are examples of notable roles in natural process. For example, the ability of geckos to climb walls and ceilings rests on the van der Waals attraction between surfaces and their foot-hairs. The energy released upon physisorption can be absorbed as vibrations of the substrate lattice (increased thermal motion) and thus lead to an approaching particle bouncing across the surface, until it has lost its energy. This process is called accommodation. The enthalpy of physisorption can be measured by monitoring the temperature of a sample of known heat capacity during the adsorption process.
Chemisorption
Atoms or molecules that stick to the surface by forming a covalent bond perform chemisorption. The enthalpy of chemisorption is much higher than in the case of physisorption (~ −200 kJ mol−1) and may thus lead to bond breakage in the adsorbate, which allows the resulting species (molecular fragments) to maximise their coordination number with the substrate. The existence of molecular fragments on the substrate is one of the reasons why solid surfaces act as catalysts.
Except for special cases, chemisorption is exothermic (ΔH < 0). An example of an endothermic process is the adsorption of H2 on glass. Since spontaneous processes require a change in the free energy of ΔG < 0, the positive enthalpy change during H2 adsorption needs to be overcome. In the case of H2 on glass, there is a significant increase in entropy as H2 molecules dissociate into H atoms, thereby outnumbering the enthalpy change and thus leading to a negative ΔG:
![]()
(2.42)
7.2.3 Adsorption Isotherms
During the adsorption process, free and adsorbed particles are in dynamic equilibrium. If we consider a gas as the adsorbing species, then the fractional surface coverage Γ depends on the pressure of the gas overlying the substrate. The variation of fractional coverage Γ with pressure p at constant temperature is called the adsorption isotherm.
If we make the following assumptions:
✵ Adsorption cannot proceed beyond monolayer coverage
✵ All sites are equivalent and the surface is uniform (at microscopic scale)
✵ The ability of a particle to adsorb is independent of the occupancy of neighbouring sites (i.e. there is no interactions between adsorbed particles)
✵ Every particle provides a single species that will be adsorbed (no dissociation)
then the rate of adsorption can then be expressed as:
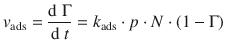
(7.13)
This equation is reminiscent of a second-order rate law. Here, the rate constant is k ads , the concentration of one reactant is given by the pressure p, and the concentration of the second reactant is the number of free sites on the substrate, [N ⋅ (1 − Γ)].
Similarly, the rate of desorption is given by:
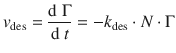
(7.14)
This is a first-order rate law, with the rate constant k des and the concentration of ’product’ given by the number of adsorbate molecules bound to substrate sites, i.e. the number of occupied sites, (N ⋅ Γ).
At equilibrium, there is no net change, so the rate of adsorption equals the rate of desorption, and by substituting expressions from Eqs. 7.13 and 7.14 one obtains:
![]()
![]()
![]()
![]()
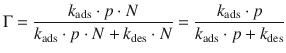
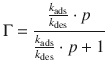
This yields an expression for the Langmuir isotherm (see Fig. 7.9), which relates the fractional coverage to the pressure of adsorbate:
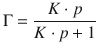
(7.15)
whereby the Langmuir constant K is given by the ratio between the rate constants of the adsorption and desorption processes:
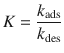
(7.16)
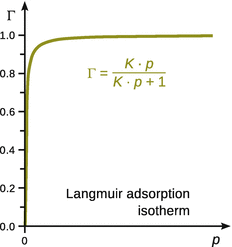
Fig. 7.9
The Langmuir adsorption isotherm describes the saturation of binding sites on the catalyst (substrate) in dependence of the pressure of gas molecules (adsorbate)
7.2.4 Adsorption Isotherms with Dissociation
When deriving the concept of the Langmuir isotherm in the previous section, we assumed that the particles arriving at the catalyst’s surface do not dissociate. In many cases (for example the synthetically important hydrogenation reactions using H2) this can not be assumed. If the adsorbate dissociates into two species upon surface attachment, then the rate of adsorption is proportional to the pressure and the probability that both fragments find sites, i.e. the square number of vacant sites. Instead of Eq. 7.13, one thus obtains:
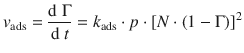
(7.17)
Similarly, for the rate of desorption, we need to take into account that two dissociated fragments re-combine and thus two occupied sites are being vacated:
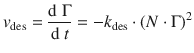
(7.18)
The combination of Eqs. 7.17 and 7.18 yields the Langmuir isotherm for adsorption with dissociation:
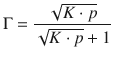
(7.19)
as illustrated in Fig. 7.10.
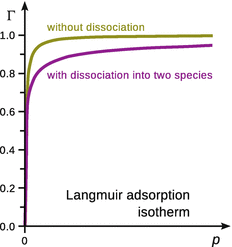
Fig. 7.10
Comparison of Langmuir isotherms for non-dissociating and dissociating (N = 2) adsorbates
7.2.5 The Isosteric Enthalpy of Adsorption
As per the definition, the isotherms describe the adsorption behaviour at constant temperature. Therefore, if adsorption experiments are carried out at different temperatures, different isotherms are obtained. The variation between those isotherms is captured by different values of ![]() . Since K is an equilibrium constant, we can use the van’t Hoff equation to obtain an enthalpy:
. Since K is an equilibrium constant, we can use the van’t Hoff equation to obtain an enthalpy:
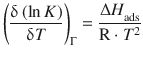
(2.60)
ΔH — ads is called the isosteric enthalpy of adsorption and is the standard enthalpy of adsorption at a fixed surface coverage.
7.2.6 Mechanisms of Heterogeneous Catalysis
The previous sections have been concerned with the adsorption of particles at the surface of a solid catalyst. Since catalysts are used to facilitate particular reactions, we now want to look at the rates of these reactions. Two different mechanisms for bimolecular surface-catalysed reactions have been proposed. The Langmuir-Hinshelwood mechanism assumes that the two molecules adsorb to the surface and then undergo the reaction. In contrast, in the Eley-Rideal mechanism, only one of the molecules adsorbs and the other one reacts with it directly from the gas phase without adsorbing. In the following, we will see that the kinetics for the two mechanisms differ. However, the rate constant k of the catalysed reaction will always be much larger than that of the non-catalysed reaction, since the reaction on the surface has a much lower activation energy.
Langmuir-Hinshelwood Mechanism
In the Langmuir-Hinshelwood mechanism, a reaction takes place between two reactants A and B adsorbed on the surface of the catalyst. The reaction rate thus depends on the extent of surface coverage by the two species, ΓA and ΓB:
![]()
(7.20)
If we use the partial pressures, p A and p B, for the two reactants, we can express the partial surface coverages ΓA and ΓB by Langmuir isotherms (assuming no dissociation):
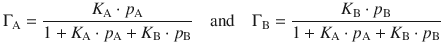
Substituting these expressions in Eq. 7.20 yields the reaction rate for the Langmuir-Hinshelwood mechanism as:
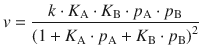
(7.21)
Examples for reactions following the Langmuir-Hinshelwood mechanism include (Table 7.2):
Table 7.2
Examples of surface-catalysed reactions that follow the Langmuir-Hinshelwood mechanism
|
Catalyst |
Reaction |
Pt |
|
Pt |
|
Pd |
|
Cu |
|
ZnO |
|
Eley-Rideal Mechanism
Examples for reactions following the Eley-Rideal mechanism include (Table 7.3):
Table 7.3
Examples of surface-catalysed reactions that follow the Eley-Rideal mechanism
|
Catalyst |
Reaction |
Pt |
|
Ni, Fe |
|
Ag |
|
In the Eley-Rideal mechanism, a reactant B in the gas phase collides with a reactant A already adsorbed on the surface. The rate of reaction is thus dependent on the partial pressure of B and the extent of surface coverage of A:
![]()
(7.22)
If the adsorption of A follows a Langmuir isotherm without dissociation, one obtains the rate of the Eley-Rideal mechanism as: