Process Technology: An Introduction - Haan A.B. 2015
3 Principles of chemical reaction engineering
3.3 Rate of chemical reactions
For the application of material balances to chemical reaction engineering problems, an expression is required for the chemical reaction rate. The reaction rate of a component is defined as the number of moles produced or consumed per unit time and volume. From this definition it follows that the chemical reaction rate is negative for reactants and positive for products. In many cases A and B are used as symbols for a reactant, and P is the symbol for a product. In the reaction equation
![]()
(3.1)
νA, νB, and νP are the stoichiometric coefficients for the respective components A, B, and P. These stoichiometric coefficients relate the relative reaction rates of the individual components:
![]()
(3.2)
It should be noted that the chemical reaction rate only reflects the chemical kinetics of the system. The reaction rate generally depends only on the composition of the reaction mixture, its temperature and pressure and the properties of the catalyst.
3.3.1 Effect of concentration
For most elementary reactions it is usually postulated that the rate-controlling mechanism involves the collision or interaction of a single molecule of A with a single molecule of B. This means that the reaction rate is proportional to the concentration of each of the reactants, since the number of molecular collisions per unit time is proportional to the amount of molecules of each of the reactants. For example, in the case of a bimolecular elementary reaction
![]()
(3.3)
the rate of disappearance of reactant A at a given temperature is related to the concentrations, CA, and CB of both reactants by the reaction rate constant, kA:
![]()
(3.4)
3.3.2 Effect of temperature
The effect of temperature on a chemical reaction is most easily explained by the transition state theory. Thermodynamics tell us that a chemical reaction will only proceed when an energetically more favorable situation is obtained. The reaction pathway towards this more stable situation usually proceeds through the formation of less stable intermediates by the breakage of chemical bonds, which subsequently decompose into the thermodynamically more stable products. This process is schematically represented in Fig. 3.1, where the potential energy of starting, intermediate and end situation is shown. From Fig. 3.1 it can be seen that activation energy, EA, has to be provided to allow a chemical reaction to proceed. In reaction rate expressions this activation energy is usually used to separate the influence of temperature and composition on reaction rate. The actual representation of the temperature, T, the dependency of the reaction rate constant, kA, is done through an exponential probability function better known as the Arrhenius relation:
![]()
(3.5)
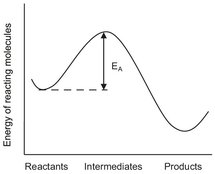
Fig. 3.1: The potential energy curve for a chemical reaction with activation energy EA.
In this equation R represents the gas constant (8.3144 J/mol K). For most reactions the activation energy lies in the range of 40-300 kJ/mol, resulting in an increase of the reaction rate constant by a factor of 2-50 for a temperature rise of 10 °C.
3.3.3 Chemical equilibria
So far it has been assumed that the reactions are irreversible. In practice, many reactions are reversible, meaning that the products can be converted back into the reactants again:
![]()
(3.6)
The rate of formation of P by the forward reaction and its rate of disappearance by the reverse reaction are now given by
![]()
(3.7)
![]()
(3.8)
Because at equilibrium there is no net formation of P, the reaction equilibrium constant, KR, can be obtained from the condition Rp,forward + Rp,reverse = 0:
![]()
(3.9)
Thermodynamics allows the calculation of the equilibrium constant from the standard free energies of formation, ![]() , of the reacting components. For the considered reaction this results in the following relation for the standard free energy of reaction,
, of the reacting components. For the considered reaction this results in the following relation for the standard free energy of reaction, ![]() , and the reaction equilibrium constant, KR:
, and the reaction equilibrium constant, KR:
![]()
(3.10)
With the equilibrium constant known, the expected maximum attainable yield of the products of the reaction can be estimated. An industrially important example where the maximum attainable conversion is limited by the thermodynamic equilibrium is the synthesis of ammonia from nitrogen and hydrogen:
![]()
(3.11)
As a result of the exothermic nature of the reaction and the volume contraction by conversion to ammonia, it is necessary to operate at relatively low temperatures and high pressures in order to achieve economically viable production rates. This is illustrated in Fig. 3.2, where the ammonia content of the equilibrium mixture is plotted as a function of pressure at various temperatures. Economically favorable conversion rates can only be obtained by catalyzing the reaction. Iron catalysts give good conversions at about 30 MPa and 500 °C in reactors of acceptable dimensions. Other typical examples of reactions in which the maximum attainable conversion is limited by the thermodynamic equilibrium are dehydrogenations (ie ethylbenzene to styrene), esterifications, and polycondensations (ie polyesters, polyamides).
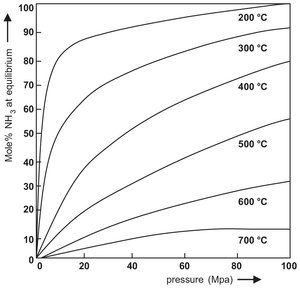
Fig. 3.2: Equilibrium yield of NH3 from N2 and H2.