Process Technology: An Introduction - Haan A.B. 2015
3 Principles of chemical reaction engineering
3.4 Catalysis
Catalysts are substances that influence the rate of a reaction without being consumed. In most cases only trace amounts are needed to bring about or accelerate a reaction or reaction type. As illustrated by Fig. 3.3, the catalyst forms complexes with the intermediates which have significantly lower activation energy to provide a reaction pathway that proceeds in parallel with the existing thermal reaction. Hence, useful catalysis implies that the rate of the desired reaction considerably exceeds the rates of all other possible reactions. If a reaction is reversible, the reverse process is accelerated to the same extent as the forward. Furthermore, a catalyst in no way alters the thermodynamic properties of reactants and products. Hence, a catalyst can only effect, or accelerate, a reaction which is thermodynamically feasible under practically attainable conditions.
Two broad classes of catalytic reactions are recognized: homogeneous and heterogeneous. When the catalytic material is in the same phase as the main reactants, for example in a solution/liquid reaction mixture, the term homogeneous catalysis is applied. The term heterogeneous catalysis is used when the catalytic material exists as a distinct, usually solid phase with liquid or gaseous reactant. Some typical examples of homogeneous and heterogeneous catalysis systems are listed in Tab. 3.2.
3.4.1 Homogeneous catalysis
In homogeneous catalysis, the catalyst is usually dissolved in a liquid reaction mixture, though some or all of the reactants may be introduced as gases, or even as solids. In general, the nature of the catalytic material used in solution chemistry is well defined, and usually highly reproducible kinetic behavior is obtained. In the case of transition metals, homogeneous catalysis has the advantage over heterogeneous catalysis where tailor-made ligands can be attached to the catalyst metal to optimize the selectivity. Fig. 3.4 illustrates that the cycle of a homogeneous catalytic reaction can be divided into four major steps:
· (1) formation of a reactive species;
· (2) coordination of the reactants to the complex;
· (3) formation of the product on the catalyst;
· (4) elimination of the formed product under liberation of the reactive starting species.
The major problem encountered in homogeneous catalysis, particularly when the catalytic material is expensive, lies in the separation of the reaction products from the catalyst such that the recovery process is efficient and does not impair catalytic activity. Thus we can afford to lose small, catalytic quantities of mineral acid, alkalis, or base metal compounds. But only minute losses can be tolerated if expensive noble-metal catalysts are used. An illustrative example is the liquid phase oxidation process for the oxidation of toluene to benzoic acid. In this process a homogeneous organic cobalt(II) complex is used to catalyze the oxidation reaction. After reaction the oxidation products are recovered and purified by evaporation and distillation leaving the cobalt catalyst in the tar residue. Extraction with water is used to recover the cobalt from the tar and recycle the catalyst system to the reactor. Besides extraction, many other separation methods, such as product and reactant evaporation, precipitation and membrane filtration, have been developed to achieve a quantitative recovery of homogeneous catalysts. A different option is to use a liquid-liquid two-phase system in the reactor, where one phase contains the catalyst and the other the reactants and the product.
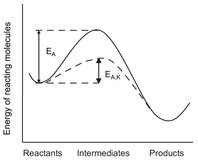
Fig. 3.3: Schematic representation of the reduction of the activation energy for a reaction by a catalyst.
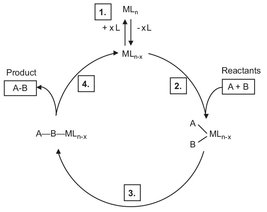
Fig. 3.4: Cycle of homogeneous catalysis.
In seeking to apply any new catalytic material, the useful lifetime of the catalyst is important. Loss of catalytic activity may be an inevitable consequence of the chemical nature of the catalytic material, or a result of poisoning. Within the first category, loss by evaporation, thermal decomposition or internal rearrangement of the catalytic material may occur when too high reaction temperatures are used. Catalyst poisoning refers to materials that do not form part of the defined process chemistry, but which gain entry into the reaction mixture and lead to permanent or temporary catalyst deactivation. In general, poisons are impurities in the chemical feedstocks or corrosion products from materials of construction.
Despite the high cost of noble metals, many mass chemicals are produced with homogeneous catalysis on a large scale. This is only possible when each molecule of the catalyst produces a large amount of product (high turnover number), and the catalyst losses are kept to an absolute minimum. Important industrial applications of homogeneous catalysis are found in isomerization, hydrogenation, oligomerization, polymerization, metathesis, oxidation, hydroformulation, and carbonylation. Typical examples are listed in Tab. 3.2.
Tab. 3.2: Some examples of industrial catalysis.
Application |
Catalyst system |
|
|
Homogeneous catalysis |
Toluene oxidation |
Cobalt |
Benzoic acid oxidation |
Copper |
|
Ziegler-Natta polymerization of polyethylene |
Ti(IV) aluminium alkyls |
|
Ethylene oligomerisation |
Ni-complexes |
|
Carbonylation of butadiene and methanol |
Pd-complexes, Rhl3/HI |
|
Acid catalyzed esterification |
Sulfuric or nitric acid |
|
|
Heterogeneous catalysis |
Ammonia synthesis |
Iron oxide |
Ammoxidation of propylene to acrylonitrile |
||
Hydrogenation of benzaldehyde to benzylalcohol |
Palladium on coal |
|
Polypropylene slurry polymerization |
3.4.2 Heterogeneous catalysis
Chemists have recognized the existence of heterogeneous catalysis for over 150 years, and practical applications have increased dramatically since the beginning of the present century. A heterogeneous catalyst is a solid composition that can effect or accelerate reaction by contact between its surface and either a liquid-phase reaction mixture or gaseous reactants. In liquid-phase systems, one or more of the reactants may be introduced as a gas, but access of such reactants to the surface of the catalyst is almost invariably by dissolution in the reaction medium and subsequent diffusion.
Heterogeneous catalysis occurs on the surfaces of solid materials. To reach this surface the reactants first have to be transported to the catalyst and — if the particles are porous — through the pores of the particle to reach the active material. The initiation of a chemical reaction involving an otherwise stable molecule requires a significant electronic disturbance in that molecule at the catalytic surface. The adsorption process that brings about such a chemical modification is usually referred to as chemisorption. Furthermore, when two or more molecules are involved in a reaction on a catalytic surface, as in hydrogenation or oxidation processes, we usually find evidence that the major reactions occur between chemisorbed or surface species derived from each of the reactant molecules. In many instances it was recognized that only a relatively small proportion of the surface was catalytically active. Hence the term active sites was introduced to describe those localities on the surface which would induce the desired chemical reaction. The combination of these complex processes makes heterogeneous catalysis one of the most complex branches of chemical kinetics. Rarely do we know the compositions, properties, or concentrations of the reaction intermediates that exist on the surfaces covered with the catalytically effective material. It should therefore be no surprise that the selection of a solid catalyst for a given reaction is to a large extent still empirical and based on prior experience and analogy.
Many heterogeneous catalysts in commercial use contain several components, often referred to as the active catalyst agent, promotor, and support. While all components contribute to the overall performance of the catalytic material, the active catalyst agent is essential for any activity in the type of reaction required. With metal catalysts, classification is usually reasonably straightforward. However, with oxide catalysts none of the components may be individually active. Promotors are introduced to modify the crystal structure or electronic properties of the major component, to improve activity, selectivity or thermal stability. As illustrated by Tab. 3.3, solid catalytic materials can be divided into two major groups:
· (1) bulk catalytic materials, in which the gross composition does not change significantly throughout the material, such as silver wire mesh or a compressed pellet of ’bismuth molybdate’ powder;
· (2) supported catalysts, in which the active catalytic material is dispersed over the surface of a porous solid, such as activated carbon, alumina, silica, or zeolites.
Bulk metals can be used in traditional engineering forms, more particularly as fine wire woven into gauzes. Such forms are generally used only in high-temperature processes, such as the partially oxidative dehydrogenation of methanol (over Ag) or ammonia oxidation (over Pt-Rh) at about 500-600 °C and 850-900 °C, respectively. Mechanical stability is of greater importance than high surface area.
Tab. 3.3: Classification of heterogeneous catalysts.
Group |
Catalyst classes |
Examples |
|
Bulk catalytic materials |
Metals |
Pt— or Ag-nets, Raney-nickel |
Metal alloys |
Pt-Re, Ni-Cu, Pt-Au |
|
Acids |
SiO2/Al2O3, zeolites, montmorillonite |
|
Bases |
CaO, K2O, Na2O |
|
|
Supported catalysts |
Oxides of transition metals |
Cr2O3, Bi2O3/MoO3, V2O5, NiO |
Oxides of other metals |
Al2O3, SiO2 |
|
Metal sulfides |
MoS2, WS2 |
|
Metals on supports |
Pt/Al2O3, Ru/SiO2, Co/kieselgur |
|
Metals and acids |
Pt/zeolite, Pd/zeolite |
|
Other |
Carbide, silicide |
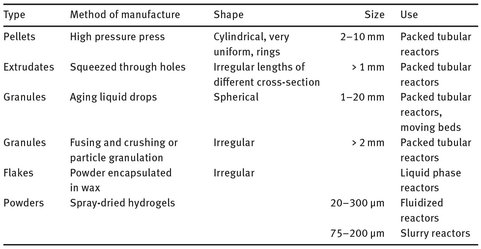
A common feature of heterogeneous catalysis is the increase in rate with increasing subdivision of the catalytic material. This arises from the increasing accessibility of the surface, and reduction in diffusional constraints between reactants and catalytic sites. The surface area, pore structure, and chemical composition of the surface are extremely important parameters of any support material or solid catalyst. For high activity at modest temperatures, forms presenting a high surface area to the reactants are highly desirable. Finely divided metal powders often show very high catalytic activity, but may present separation problems. Hence, we find the development of methods to produce coarser particles of metals in porous form, such as platinum sponge or, far more commonly, Raney nickel. Alternatively, larger shaped catalysts in the shape of cylinders, spheres, rings, etc. can be produced from powder, by compression into moulds or extrusion of a slurry and drying. An overview of the most common catalyst particles and production techniques is given in Tab 3.4.
Tab. 3.4: Common catalyst particles.