Process Technology: An Introduction - Haan A.B. 2015
3 Principles of chemical reaction engineering
3.7 Heat effects in model reactors
Chemical reactions are always accompanied by energy effects. The amount of heat liberated or absorbed during reaction depends on the nature of the reacting system, the amount of material reaction, and is calculated from the heat of reaction ΔHr under the operating conditions. When this is not known, it can in most cases be calculated from known and tabulated thermochemical data on formation heat or combustion heat of the reacting chemicals. Heat-producing reactions are called exothermic, and reactions where the addition of heat is required is called endothermic.
3.7.1 Energy balances
In nonisothermal operations, energy balances must be used in conjunction with material balances. The general form of the heat balance for a reactor has a form very similar to the general material balance:

(3.52)
In the inflow and outflow terms, the heat flow may be of two kinds. The first is the transfer of sensible heat or enthalpy Q (J/s) by the fluid entering and leaving, calculated from the density of the fluid ρ (kg/m3) and the specific heat of the fluid Cp (J/kg · K) according to
![]()
(3.53)
The second is the heat transferred to or from the fluid across heat-transfer surfaces, such as cooling coils inside or jackets outside the reactor. In many cases the temperature TC on the cooling or heating side can be taken to be approximately constant, and the rate of heat transfer Q across a surface of area A can be written as
![]()
(3.54)
where T is the temperature of the reaction mixture, and U (W/m2 · K) is the heat-transfer coefficient. Combining all the results in the following general heat balance, which can be applied to a stirred tank reactor or an element from a tubular reactor, we have
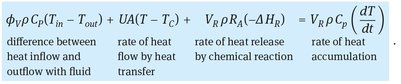
(3.55)
The rate of heat production depends on the rate of reaction, which in turn depends on the concentration levels in the reactor as determined by the general mass balance equation. Since the rate of reaction also depends on the temperature levels in the reactor, the material and heat balances interact strongly with each other and have to be solved simultaneously.
3.7.2 Characteristics of a continuous stirred tank reactor
For a stationary continuous stirred tank reactor, this results in the characteristic behavior schematically shown in Fig. 3.15. As the temperature increases, the reaction rate RA increases rapidly according to the Arrhenius relation, but then tends to an upper limit as the reactant concentration in the tank approaches zero, corresponding to almost complete conversion. On the other hand, the rate of heat removal by both product outflow and heat transfer is virtually linear, as shown in Fig. 3.16. To satisfy the heat balance equation, the point representing the actual operating temperature is obtained from the intersection of the heat production curve and the rate of heat removal line. If the rate of heat removal is high, due either to rapid outflow or to a high rate of heat transfer, there is only one point of intersection, corresponding to a low operating temperature. With a somewhat smaller heat removal there are three points of intersection corresponding to two stable operating conditions. The intermediate point of intersection is an unstable operating point where the slightest process disturbance will cause the system to pass into the lower or upper operating state. At an even lower heat transfer, only an operating temperature corresponding to nearly complete conversion is possible.
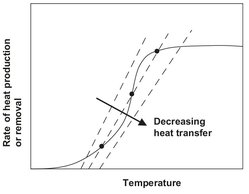
Fig. 3.15: Heat production and removal for a continuous stirred tank reactor.
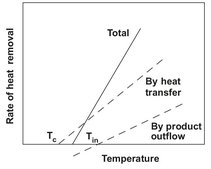
Fig. 3.16: Rate of heat removal from a continuous stirred tank reactor.
3.7.3 Batch reactors
The balance between the rate of heat production and rate of heat removal or addition determines the temperature profile of a batch reactor is as follows:
![]()
(3.56)
In general the simultaneous solution of the material and energy balances for a batch reactor is only possible by numerical simulation. For an exothermic reaction, the reactor temperature usually rises after starting the reaction. A typical requirement is that the temperature does not rise above the maximum allowable temperature to avoid by-products or hazardous operation. This is achieved by using sufficient cooling, as illustrated by Fig. 3.17.
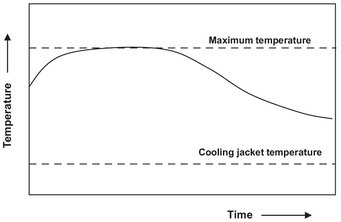
Fig. 3.17: Typical reactor temperature profile for an exothermic reaction in a nonisothermal batch reactor with just sufficient cooling.
Nomenclature
A |
surface area |
[m3] |
C |
concentration |
[mol m-3] |
Cp |
specific heat |
[J kg-1 K-1] |
EA |
activation energy |
[J mol-1] |
Δ G° |
standard free energy |
[J mol-1] |
ΔH° |
standard enthalpy |
[J mol-1] |
k |
reaction rate constant (unit depends on reaction, first order=) |
[mol s-1] |
k∞ |
pre-exponential factor (unit depends on reaction, first order=) |
[mol s-1] |
KR |
reaction equilibrium constant |
[-] |
L |
reactor length |
[m] |
N |
number of moles |
[mol] |
N |
number of reactors |
[-] |
Ptot |
total pressure |
[N m-2] |
Q |
enthalpy |
[J s-1] |
R |
reaction rate |
[mot s-1] |
R |
gas constant (8.3144) |
[J mol-1 K-1] |
S |
axial surface area |
[m2] |
t |
time |
[s] |
T |
temperature |
[K] |
U |
heat transfer coefficient |
[W m-2 K-1] |
v |
longitudinal velocity |
[m s-1] |
VR |
volume of reaction, reactor volume |
[m3] |
z |
position in tubular reactor |
[m] |
φv |
volumetric flow rate |
[m3 s-1] |
η |
yield |
[-] |
ν |
stoichiometric coefficients |
[-] |
p |
density |
[kg m-3] |
σ |
selectivity |
[-] |
r |
reaction time, residence time |
[s] |
ξ |
degree of conversion |
[-] |
Indices
A |
reactant |
B |
reactant |
C |
cooling water |
F |
formation |
K |
intermediate complex |
0 |
initial (time=0) |
P |
product |
R |
reaction |