Process Technology: An Introduction - Haan A.B. 2015
7 Extraction and leaching
7.1 Liquid-liquid extraction
7.1.1 Introduction
Since the introduction of industrial liquid-liquid extraction processes, a large number of applications have been proposed and developed. An overview of some important industrial example applications is presented in Tab. 7.1. The first and until now largestvolume industrial application of solvent extraction is in the petrochemical industry. Extraction processes are well suited to the petroleum industry because of the need to separate heat-sensitive liquid feeds according to chemical type (e.g. aliphatic, aromatic, naphthenic) rather than by molecular weight or vapor pressure. Other major applications include the purification of antibiotics and the recovery of vegetable oils from natural substrates. Some applications in metal processing are the recovery of metals such as copper from acidic leach liquors and the refining of uranium, plutonium, and other radioactive isotopes from spent fuel elements. Recently extraction is gaining increasing importance as a separation technique in biotechnology.
Tab. 7.1: Industrial liquid-liquid extraction processes.
Solute |
Carrier |
Solvents |
Acetic acid |
Water |
Ethyl acetate, isopropyl acetate |
Aromatics |
Paraffins |
Diethylene glycol, furfural, sulpholane, NMP, DMSO |
Caprolactam |
Aqueous ammonium sulphate |
Benzene, toluene, chloroform |
Benzoic acid |
Water |
Benzene |
Formaldehyde |
Water |
Isopropyl ether |
Phenol |
Water |
Benzene |
Penicillin |
Broth |
Butyl acetate |
Vanilla |
Oxidized liquors |
Toluene |
Vitamins A, D |
Fish liver oils |
Liquid propane |
Vitamin E |
Vegetable oils |
Liquid propane |
Copper |
Acidic leach liquors |
Chelating agents in kerosene |
Uranium |
Acidic leach liquors |
Tertiary amines in kerosene |
Liquid-liquid extraction is based on the partial miscibility of liquids and used to separate a dissolved component from its solvent (carrier) by transfer to a second solvent. The principle of liquid-liquid extraction, and its special terminology is illustrated in Fig. 7.1. In the simplest case the feed solution consists of the carrier solvent containing the desired solute. This liquid feed is contacted with a solvent, which is immiscible or only partly miscible with the liquid feed. In general the solvent has a higher affinity for the solute than the carrier and the solute is extracted into the solvent phase.
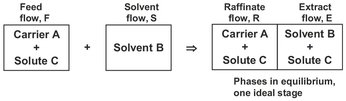
Fig. 7.1: Principle of a single extraction contacting stage.
For efficient contact a large interfacial area must be created across which the solute can transfer until equilibrium is closely approached. This is achieved by bringing the feed mixture and the solvent into intimate contact. When equilibrium is reached, the stage is defined as an ideal or theoretical stage and the equilibrium conditions can be expressed in terms of the extraction factor E for the solute:
![]()
(7.1)
where S and F are the solute free solvent and feed streams, respectively, and K is the distribution coefficient of the solute between the solvent and the carrier. The larger the value of E, the greater is the extent to which the solute is extracted. Large values of E result from large values of the distribution coefficient, K, or large solvent to carrier ratios. The resulting solute loaded solvent phase is called the extract, while the other liquid phase is designated as the raffinate.
After extraction at least one distillation column (or other separation process) is required to separate the solvent from the extract and recycle the solvent. If the solvent is partially miscible with the feed, a second separation process (normally distillation) is required to recover solvent from raffinate. Fig. 7.2 illustrates such an extraction system where two distillation columns are needed. This example is based on a high-boiling solvent, which is recovered as the bottom product of the distillation column. A low-boiling solvent would be recovered as the top product. Solvent recovery is an important factor in the economics of industrial extraction processes. Especially if the solvent and solute have close boiling points, the distillation column may require many trays and a high reflux ratio, resulting in a costly process.
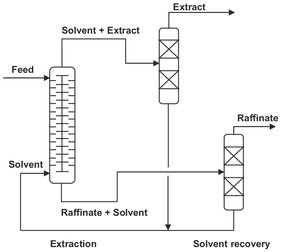
Fig. 7.2: Schematic of a liquid-liquid extraction process.
The main disadvantage of extraction is that the necessity of a solvent increases the complexity and thereby costs of the process. Situations where extraction is preferred to distillation include the following application areas:
· — dissolved or complexed inorganic substances in organic or aqueous solutions;
· — removal or recovery of components present in small concentrations;
· — when a high-boiling component is present in relatively small quantities in a waste steam;
· — recovery of heat-sensitive materials and low to moderate processing temperatures are needed;
· — separation of a mixture according to chemical type rather than relative volatility;
· — separation of close-melting or close-boiling liquids, when solubility differences can be exploited;
· — mixtures that form azeotropes or exhibit low relative volatilities and distillation cannot be used.
7.1.2 Liquid-liquid equilibria
Addition of a solvent to a binary mixture of the solute in a carrier can lead to the formation of several mixture types. The simplest ternary system is the Type I system for one immiscible-liquid pair shown in Fig. 7.3. In such a system, the carrier and the solvent are essentially immiscible, while the solute is miscible with the carrier as well as the solvent. Such ternary systems are commonly represented in a triangular diagram, showing the two-phase envelope that encloses the region of overall compositions in which two phases exist in equilibrium. The equilibrium compositions of the extract and raffinate phases are connected by tie-lines that can be used to determine distribution coefficients and selectivities. For each component the distribution coefficient is given by the ratio of the concentrations in the two phases as
![]()
(7.2)
where xi,E is the mole fraction of component i in the extract phase, and xi,R is the mole fraction of component i in the raffinate phase. The selectivity is, equivalent to the relative volatility in distillation, defined as the ratio of K values:
![]()
(7.3)
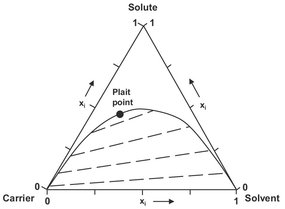
Fig. 7.3: Triangular diagram for a Type I system with the tie lines represented by the dashed lines.
At equilibrium, thermodynamics requires the activity of each component to be the same in the two liquid phases. However, due to the strong thermodynamic nonideal behavior of the two liquid phases, the large difference in activity coefficients causes significant differences in equilibrium compositions:
![]()
(7.4)
Replacing the composition ratio with the ratio of activity coefficients gives for the selectivity
![]()
(7.5)
The required activity coefficients or distribution coefficients are generally calculated from thermodynamic models such as NRTL, UNIQUAC, or predicted from group contribution methods like UNIFAC. In Fig. 7.3 it can be seen that as the concentration of the solute is increased, the tie-lines become shorter because of the increased mutual miscibility of the two phases. At the plait point the raffinate-phase and extract-phase boundary curves intersect and the selectivity becomes equal to one. An important additional use of the triangular diagram is the graphical solution of material balance problems, such as the calculation of the relative amounts of equilibrium phase obtained from a given overall mixture composition. Liquid-liquid equilibria having more than three components cannot as a rule be presented in a two-dimensional diagram.
7.1.3 Solvent selection
The key to an effective extraction process is the selection of the right solvent. A solvent or extractant may be a pure chemical compound or a mixture of chemical compounds. Proposing a solvent requires the knowledge of the physical and chemical properties of the solvent, solute, carrier, and other constituents of the extraction system. While pure component properties are easily found in the literature, mixture physical properties are available in the literature for very few systems. The following criteria are important to consider during the selection of a solvent:
· — Distribution coefficient — A high value of distribution coefficient indicates high affinity of the solvent for the solute and permits lower solvent/feed ratios.
· — Selectivity — A high value of the selectivity reduces the required number of equilibrium stages. If the feed is a complex mixture where multiple components need to be extracted from, selectivities between groups of components become important.
· — Density — Higher density differences between extract and raffinate phases permits higher capacities in extraction devices using gravity for phase separation. The density difference must at least be large enough to ease the settling of the liquid phases.
· — Viscosity — High viscosities lead to difficulties in pumping, dispersion, and reduces rate of mass transfer. Low viscosities benefit rapid settling and capacity.
· — Solvent recoverability — Recovery of the solvent should be easy. A solvent which boils much higher than the solute generally leads to better results, though solvents boiling lower than the solute are also used commercially.
· — Solubility of solvent — Mutual solubilities of carrier and solvent should be low to avoid an additional separation step for recovering solvent from the raffinate.
· — Interfacial tension — Low interfacial tension facilitates the phase dispersion but may require large volumes for phase separation due to slow coalescence. High interfacial tension permits a rapid settling due to an easier coalescence, allowing higher capacities. A too low interfacial tension leads to emulsification.
· — Availability and cost — Solvent cost may represent a large initial expense for charging the system, as well as a heavy continuing expense for replacing solvent losses. Therefore, one should make sure that the solvent of interest is commercially available and relatively inexpensive.
· — Toxicity, compatibility and flammability — These criteria are important occupational health and safety considerations which a suitable solvent has to meet. Especially for food and pharmaceutical products only nontoxic solvents will be taken into consideration. In general, any hazard associated with the solvent will require extra safety measures and increases costs.
· — Thermal and chemical stability — It is important that the solvent should be thermally and chemically stable because it is recycled. In particular the solvent should resist breakdown during recovery in for example a distillation column.
· — Corrosivity — Corrosive solvents can lead to increased equipment cost but might also require expensive pre— and posttreatment of streams.
· — Environmental impact — The solvent should not only be compatible with the process, but also with the environment (minimal losses due to evaporation, solubility, and entrainment)
In addition to being nontoxic, inexpensive, and easily recoverable, a good solvent should be relatively immiscible with feed components other than the solute and have a different density from the feed to facilitate phase separation. Also, it must have a very high affinity for the solute, from which it should be easily separated by distillation, crystallization, or other means. Obviously no solvent will be best from all of these viewpoints, and the selection of a desirable solvent involves compromises between the various criteria. In this selection process some or the criteria are essential for the separation, while others are desirable properties that will improve the separation and/or make it more economical. The most important compromise is always between solute solubility and selectivity that usually behave in exactly opposite fashion. When a high selectivity is obtained, solubilities are usually low, and vice versa. In most cases, the selectivity is the most important parameter, since this determines whether a certain separation can be accomplished.
7.1.4 Extraction schemes
In the simplest extraction scheme a feed and solvent are contacted as shown in Fig. 7.1. In the case that the solvent and the carrier are completely immiscible and the solvent contains no solute, the material balance over the solute becomes
![]()
(7.6)
which can be rearranged by elimination of xE = K xR to give
![]()
(7.7)
The main disadvantage of single-stage contacting is that residual solute is left behind in the carrier. Therefore often a series of contacting stages is arranged in a cascade to accomplish a separation which cannot be achieved in a single stage and/or reduce the required amount of the mass-separating agent. Multistage liquid-liquid extraction cascades can be arranged in a cocurrent, crosscurrent, and countercurrent arrangement, as shown in Fig. 7.4. When the stages are ideal, cocurrent stage-wise contact is not necessary, because equilibrium is reached between the streams after the first stage.
A crosscurrent cascade in which the fresh solvent distributed over multiple stages gives an improvement over the separation obtainable in a single stage for a given ratio of solvent to feed. Since the total amount of solvent is distributed over N stages, the extraction factor for each stage becomes E/N, giving for the raffinate concentration after N stages:
![]()
(7.8)
Thus unlike the cocurrent cascade, the value of xN decreases in each successive stage. The best compromise between the objectives of high extract concentration and a high degree of extraction of the solute is the countercurrent arrangement. The feed entering Stage I is brought into contact with a solute-rich stream which has already passed through the other stages, while the raffinate leaving the last stage has been in contact with fresh solvent. Because of economic advantages, continuous countercurrent extraction is normally preferred for commercial scale operations. For a countercurrent operation the raffinate concentration after N stages is given by
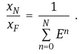
(7.9)
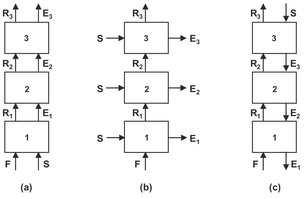
Fig. 7.4: Schematic of cocurrent (a), crosscurrent (b), and countercurrent (c) extraction.
As with the crosscurrent arrangement, the value of xN decreases in each successive stage. The decrease for the countercurrent arrangement is larger than for the crosscurrent arrangement, and the difference increases exponentially with increasing extraction factor, E. Therefore the countercurrent cascade is the most efficient of the three linear cascades. In the case that the solvents are substantially immiscible and the distribution coefficient remains constant, the number of theoretical stages, NS, for countercurrent contact can be calculated from the Kremser equation:
![]()
(7.10)