Process Technology: An Introduction - Haan A.B. 2015
7 Extraction and leaching
7.3 Leaching
Many natural substances occur in a mixture of components dispersed through a solid material. The separation of a desired soluble constituent from its solid by means of a solvent is referred to as leaching. Originally the term leaching was used to describe the process of percolating a liquid through a solid material. During those days leaching was carried out mainly as a batch process but nowadays many continuous plants have been developed.
Liquid-solid extraction covers a variety of processes such as the extraction of oil from seeds with hexane and sugar from beet or coffee from ground roasted coffee beans with hot water. Leaching is often accompanied by a chemical reaction, which converts the extract into soluble form. Examples are the hydrolysis of wood and many leaching processes used in metallurgy and the pigment industries. A list of industrial leaching operations is given in Tab. 7.2.
7.3.1 Mechanism and process of leaching
If the solute is uniformly dispersed in the solid, the material close to the surface dissolves first, leaving a porous structure in the solid residue. The solvent will then have to penetrate this outer porous region before it can reach further solute. The process becomes progressively more difficult and the rate of extraction will decrease. If the solute forms a large proportion of the volume of the original particle, its removal can destroy the structure of the particle that may crumble away. In such cases the solvent easily accesses further solute and the extraction rate does not fall as rapidly. Sometimes the soluble material is distributed in small isolated pockets in a material that is impermeable to the solvent. An example is gold dispersed in rock that is crushed to expose all the soluble material to the solvent. The limiting extraction rate is influenced by four important factors:
· — Solid particle size — A smaller particle size increases the relative interfacial area between the solid and liquid and the extraction rate. However, smaller particle sizes tend to lead to lower drainage rates from the solid residue and can create problems in the solids flow through countercurrent extraction equipment.
· — Solvent — The liquid chosen should be selective and its viscosity should be sufficiently low to allow free circulation.
· — Temperature — In general the solute solubility and rate of extraction increase with temperature.
· — Agitation of fluid — Agitation of the solvent improves the mass transfer of material form the surface of the particles to the bulk of the solution.
Traditionally that part of the solvent retained by the solid is referred to as the underflow or holdup, whereas the solid-free solute-rich solvent separated from the solid after extraction is called the overflow. The solid can be contacted with the solvent in a number of different ways. Countercurrent extraction offers the most economical use of solvent, permitting high concentrations in the final extract and high recovery from the initial solid. In a multistage operation fresh solid enters the first stage and fresh solvent enters the final stage. The solvent is gradually enriched in solute until it leaves the extraction battery as overflow of from the first stage. The operation is usually discontinuous in that the solvent is pumped from one vessel to the next intermittently and allowed to remain until equilibrium extraction is approached. A complete solidliquid extraction process consists of the following stages:
· — preparation of the feed material in such a way that the solvent can dissolve the extract quickly: this is achieved by crushing, grinding or flaking;
· — contact of liquid solvent with the solid to effect transfer of solute to the solvent
· — separation of resulting solution from the residual solid;
· — recovery of the extracted solute from the solvent;
· — separation of solvent residues from the extracted solid.
In leaching processes, special attention is needed for solvent regeneration and solute recovery. Solvent recovery is often energy intensive, and a full process energy analysis is recommended to reduce costs. Recovery of organic solvents from the exhausted solids is also important and can be more troublesome than recovery from a liquid.
7.3.2 Solid-liquid extractors
Extractors often contribute substantially to the capital and operating costs of a plant. They use either percolation or agitation to ensure intimate contact between the solids and solvent. In a percolation system, extraction rate needs to be high, as the solvent residence time is often relatively short. A percolation process can be carried out in either stagewise or in a differential contactor. For an immersion process stagewise contact is often more practicable, especially when a low extraction rate requires a long residence time or multiple contact with the solvent.
7.3.2.1 Batch extractors
Batch extraction is based on the principle of displacement or enrichment. In the displacement method a given quantity of fresh solvent is added to the feed material. After thorough mixing, the extraction residue is allowed to settle and the loaded solvent (extract) is drawn off. The procedure is repeated until the required degree of extraction has been achieved. This system, known as cross flow extraction, is normally carried out in agitated vessels, as shown in Fig. 7.10. Filling and emptying takes place through the top of the vessel. After extraction the solvent is distilled of from the residue by direct heating of the extractor.
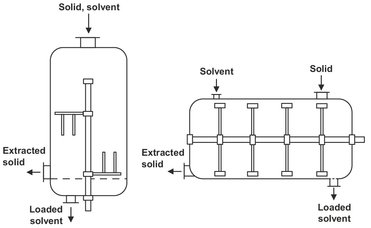
Fig. 7.10: Vertical and horizontal agitated extraction vessels.
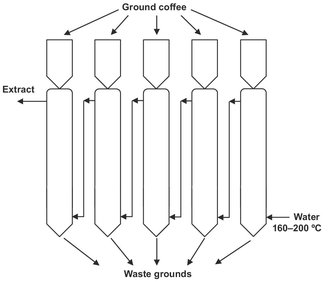
Fig. 7.11: Batch percolation battery for soluble coffee extraction.
In the enrichment method several batch extractors are connected to form a battery operating on a cyclic basis. The vessels are charged with the solids to be extracted and the solvent is passed counter currently through the extraction system. The fresh solvent flows into the extractor containing the fully extracted material, and becomes increasingly enriched with extract as it flows through the following stages. In the cyclic operation, the most exhausted diffuser is bypassed and emptied, and an empty one is charged with fresh solids. This plant layout, schematically shown in Fig. 7.11, is used for the extraction of coffee solubles using hot water.
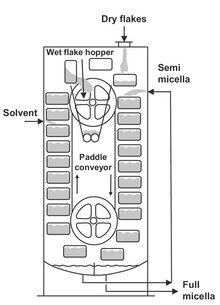
Fig. 7.12: The Bollman extractor.
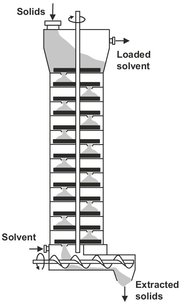
Fig. 7.13: The Bonotto extractor.
7.3.2.2 Continuous extractors
Continuous extractors are available in a variety of forms. The main difference between them is the way by which the solids are transported through the equipment. Only a few characteristic types are described here. Moving-bed percolation systems are used for extraction from many types of cellular particles such as seeds, beans and peanuts. An example is the Bollman extractor shown in Fig. 7.12. The solids are loaded into perforated baskets fixed to a chain conveyor in a closed vessel as in a bucket elevator. Solid is fed to the top basket on the downward side of the conveyor and is discharged from the top basket on the upward side. Fresh solvent is sprayed on the solid about to be discharged and passes downward through the baskets to effect a countercurrent flow. An alternative tower design, the Bonotto extractor, consists of a tall cylindrical vessel with a series of slowly rotating horizontal trays (Fig. 7.13). The solid is fed continuously on the top tray and a stationary scraper moves it towards the center of the tray. The solid then falls through an opening onto the tray beneath, where another scraper moves the solid across the tray in the opposite direction. The solvent is fed to the bottom of the vessel and flows upward to give a flow countercurrent to the solids flow direction.
Endless belt percolation extractors are similar to a belt filter. Fig. 7.14 shows that they are fitted with a slow-moving perforated belt on which the solid is fed from a hopper. Fresh solvent is sprayed onto the bed close to the discharge end of the belt. The collected solvent is sprayed back onto the bed at a point closer to the solids-feed end of the belt. This process is repeated to achieve extraction with a countercurrent flow. The extraction and percolation rates determine the belt speed, amount of drainage area, and hence the length of the belt required.
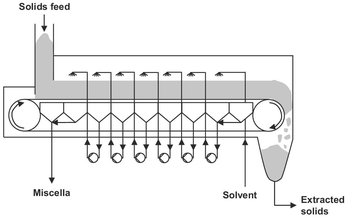
Fig. 7.14: Schematic of the belt-type extractor.
Immersion extraction systems are useful in handling finely ground material or when the percolation rate through the material to be extracted is too rapid to allow effective extraction from the solids. They have been made continuous through the inclusion of screw conveyors to transport the solids. A relative simple version of these machines is the De Danske Sukkerfabriker diffuser extractor shown in Fig. 7.15. A double screw is used to transport the solids up the gradient of the shell, while the solvent flows counter currently down the gradient.
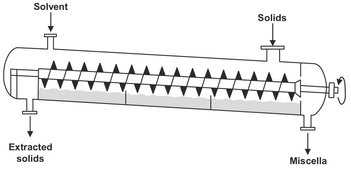
Fig. 7.15: The De Danske Sukkerfabriker extractor.