Process Technology: An Introduction - Haan A.B. 2015
7 Extraction and leaching
7.4 Supercritical extraction
7.4.1 Introduction
Supercritical extraction is a separation process that applies a supercritical fluid as a separating agent in the same way as for instance, liquid solvents are used in extraction or absorption. The supercritical region of a pure fluid is defined as the area above the critical pressure Pc and critical temperature Tc, as shown by the crosshatched area in Fig. 7.16. In this area the operating temperature is above the critical temperature and the operating pressure above the critical pressure of the solvent. The region in the immediate vicinity of the critical point is called the critical fluid region and the region just under the critical point is the near critical region.
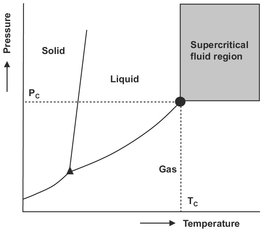
Fig. 7.16: Schematic pressure-temperature diagram for a pure substance showing the supercritical fluid region.
Supercritical fluids may be used in the same way as other ordinary solvents, although it can be advantageous to take into account their somewhat different behavior. The main difference is that above the critical temperature the discontinuity between liquid and vapor disappears, enabling a continuous transition by isothermal compression form a dilute phase to a dense phase, without passing through a two-phase region.
7.4.2 Properties of supercritical fluids
The solvent strength of a given supercritical compressed fluid is related directly to the fluid density and extremely sensitive to pressure and temperature near the critical point. This effect of pressure on the solubility in the vicinity of the critical point can be explained by considering the density behavior of a pure solvent, shown in Fig. 7.17. For reduced temperatures (T/Tc) ranging from 1.0 to 1.2, small changes in reduced pressure (P/Pc) can change the reduced density (ρ/ρc) of a solvent from about 0.1, a gas-like density, to about 2.5, a liquid-like density. This means that by adjusting the pressure and/or temperature, the properties of a supercritical solvent can be changed from a gas, with little solvating power, to a liquid, with good solvating power. At higher reduced temperatures, Tr > 1.5, the same variation in density can only be achieved by a much larger increase in pressure. Similarly, a significant increase in density can be obtained with only a small decrease in temperature for a reduced pressure Pr close to unity, while for Pr = 7 the effect of temperature on the density is much smaller.
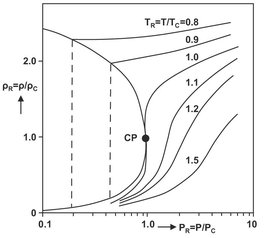
Fig. 7.17: Variation of the reduced density of a pure component in the vicinity of its critical point (CP).
Thus, solvent strength may be manipulated over a wide range by making small changes in temperature and pressure. Based on this principle, there are three possible operation modes of supercritical extraction processes. These operation modes are illustrated in Fig. 7.18, showing the solubility of naphthalene in supercritical carbon dioxide as a function of pressure and temperature. Depending upon the pressure level, the temperature is seen to affect the naphthalene solubility quite differently. For example, at 300 atm an increase in the temperature increases the solubility of naphthalene in carbon dioxide, whereas at a low pressure of 80 or 90 atm an increase in temperature decreases the naphthalene solubility. Most commonly used is the combination of extraction at elevated pressure with separation of solvent and extract by pressure reduction (a). At very high operating pressures this configuration becomes uneconomical due to the large recompression costs. In that case isobaric cooling of the solvent (b) will separate the extract because the solubility depends mainly on the vapor pressures of the solutes. An opposite effect of temperature on the solubility occurs when operating close to the critical pressure of the solvent. Isobaric heating of the solvent (c) will reduce the solubility as a result of a large decrease in solvent density.
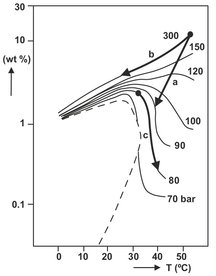
Fig. 7.18: Schematic representation of the three basic process operations in a solubility diagram for naphthalene in carbon dioxide.
Tab. 7.3: Comparison of some physical properties for a gas, supercritical solvent, and a liquid.
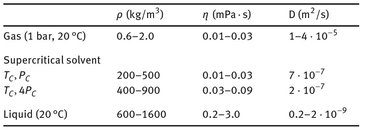
Tab. 7.3 compares some physical properties of a gas, a supercritical solvent, and a liquid. It is seen that although a supercritical solvent has a liquid-like density, the viscosity is more like that of a gas, resulting in diffusion coefficients that are much higher than diffusion coefficients in liquids. This makes the supercritical fluid a very mobile phase, capable of faster and better penetration into a solid matrix containing substances to be extracted.
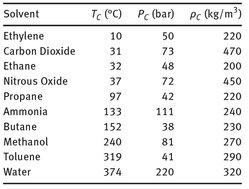
A list of the critical temperatures, pressures and densities of some gases and liquids is given in Tab. 7.4. The solvents of interest for most of the practical applications have moderate critical temperatures ranging from 0 to 100 °C. For large-scale applications, propane, ethane, and carbon dioxide are the most suitable solvents, because they are cheap, readily available, and nontoxic. It is clear that carbon dioxide is the most attractive solvent because it is nonflammable and therefore easy to handle. Water has an unusually high critical temperature owing to its polarity. At supercritical conditions water can dissolve gases such as oxygen and nonpolar organic compounds.
Tab. 7.4: Critical properties for common supercritical solvents.
7.4.3 Processes and applications
A generalized process scheme for the production of an extract from a starting mixture with supercritical CO2 is shown in Fig. 7.19. The supercritical fluid extracts the desired components in the extraction vessel. The fluid phase is expanded in a pressurereducing valve, and because of the decrease in the density of the CO2, the extract separates from the fluid phase and is collected in the separator. The CO2 is recompressed after being liquefied in the condenser and finally heated up to the desired extraction temperature before reentering the extraction vessel.
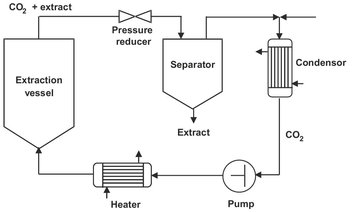
Fig. 7.19: Schematic representation of the supercritical extraction process.
Already for some decades several commercial processes have used the unique dissolving properties of solvents near their critical point. In the propane deasphalting process lube oils are refined with near-critical propane, as shown in the schematic diagram in Fig. 7.20. In the asphalt settler, saturated liquid propane at approximately 50 °C dissolves all the constituents of a lube-oil feedstock except for the asphalt. Heating the remaining propane-oil mixture to temperatures near 100 °C decreases the solvent power of liquid propane, resulting in the sequential precipitation of the resins, thus leaving only the lightest paraffins in solution.
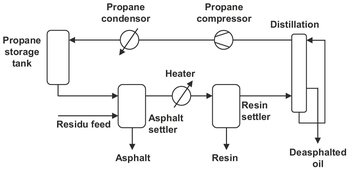
Fig. 7.20: Schematic diagram of the propane-lube oil refining process.
Another important application, the ROSE process, employs near-critical butane or pentane for the separation of heavy components such as asphaltenes, resins, and oils from oil residues. In the process (Fig. 7.21), residuum and pentane are mixed and the soluble resins and oils are recovered in the supercritical phase. By stepwise isobaric temperature increases, which decrease the solvent density, the resin and oil fractions are sequentially precipitated.
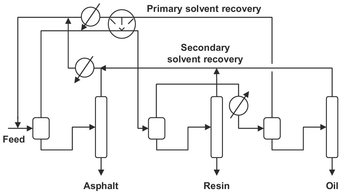
Fig. 7.21: Schematic diagram of the ROSE process.
Supercritical carbon dioxide is well known for its ability to extract thermally labile food components at near-ambient temperature without contaminating the food product. In this area decaffeination by supercritical CO2 has become the major industrial application. By using water-saturated CO2 as a solvent at a temperature around 60 °C and pressure around 300 bar, the caffeine content of coffee beans can be reduced from 3 to nearly 0.2 wt%. Fig. 7.22 shows a part of the process for green coffee bean decaffeination. Saturation of both the green (unroasted) coffee beans and the CO2 with water has been found to improve the caffeine extraction rates. Also, increasing the temperature and pressure improves the partitioning of caffeine into the supercritical phase. A large excess of carbon dioxide is required, because caffeine does not dissolve to its solubility limit during the extraction of coffee beans.
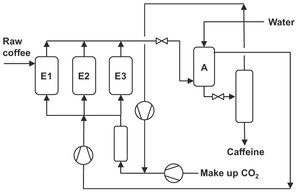
Fig. 7.22: Semicontinuous counter current supercritical coffee decaffeination.
In principle the separation of caffeine from carbon dioxide can be achieved by reducing pressure. However, pressure reduction must be substantial and is only of use if the loading of caffeine in carbon dioxide is high. For the decaffeination of green coffee beans and tea leaves this is not the case, as it would lead to high costs for recycling the CO2. Therefore alternative techniques are used to separate the caffeine isobaric from the supercritical CO2. One option is to adsorb caffeine onto activated carbon, by which high loadings are achieved. The advantage of adsorption over the classical pressure reduction is that energy consumption is reduced. Alternatively absorption in water is feasible, since the equilibrium concentration of caffeine in water is much higher than that in the gaseous phase. Absorption in water has the additional advantage over adsorption that the extracted caffeine can be recovered and sold as an additional product. Both techniques, absorption in water and adsorption on activated carbon, are currently used in commercial processes for decaffeination.
In supercritical decaffeination processes multistage countercurrent contacting is the most effective mode. It reduces the amount of solvent and enables the continuous production of extract. Real countercurrent contact is not easily established for solids, since special effort is necessary to move the solid, with increased difficulties at elevated pressures. Therefore, it is easier not to move the solid material and to achieve countercurrent contact by other measures. For the purposes of extraction from solids with supercritical solvents, several fixed beds in countercurrent contact with the solvent are the best configuration.
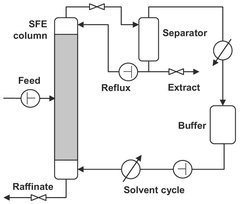
Fig. 7.23: Schematic of an installation for counter current extraction of liquids with supercritical fluids.
In the food industry carbon dioxide is also used to extract α-acids from hops. These acids impart a characteristic bitter taste to beer. Although the yields are similar to those using methylene chloride, the color, composition, odor, and texture of the extract are better controllable. Many other flavors, spices, and fragrances have been extracted. Supercritical solvents can also be used to fractionate liquid mixtures such as glycerides, vegetable oils, waxes, and polymers into numerous components. In that case the extraction is typically carried out in countercurrent columns, as illustrated schematically in Fig. 7.23. The main part of the scheme is the column where the countercurrent contact of the liquid and supercritical solvent takes place. The column is usually equipped with internals such as packing or trays to intensify the contact between both phases.
Nomenclature
A, B, C |
components |
|
CP |
critical point |
[—] |
D |
diffusion coefficient |
[m2 s-1] |
E |
extraction factor |
[—] |
E, F, R, S |
extract, feed, raffinate, solvent flow |
[amount s-1] |
Ki |
distribution coefficient of component i |
[—] |
Ns |
number of theoretical stages |
[—] |
N |
number of stages |
[—] |
P |
pressure |
[bar] |
T |
temperature |
[K] |
x |
mole or weight fraction |
[-] |
β12 |
selectivity of component 1 over component 2 |
[-] |
ρ |
density |
[kgm-3] |
η |
viscosity |
[mPa s] |
y |
activity coefficient |
[-] |
Indices
1,2, i |
components |
C |
critical |
E, F, R, S |
extract, feed, raffinate, solvent flow |
R |
reduced |