Process Technology: An Introduction - Haan A.B. 2015
8 Absorption and stripping
8.3 General design approach
Design or analysis of an absorber (or stripper) requires consideration of a number of factors, including:
· (1) entering gas (liquid) flow rate, composition, temperature, and pressure;
· (2) desired degree of recovery of one or more solutes;
· (3) choice of absorbent (stripping agent);
· (4) operating pressure and temperature, and allowable gas pressure drop;
· (5) minimum absorbent (stripping agent) flow rate and actual absorbent (stripping agent) flow rate as a multiple of the minimum rate needed to make the separation
· (6) number of equilibrium stages;
· (7) heat effects and need for cooling (heating);
· (8) type of absorber (stripper) equipment;
· (9) height of the absorber (stripper);
· (10) diameter of the absorber (stripper);
The initial step in the design of the absorption system is selection of the absorbent and overall process to be employed. There is no simple analytical method for accomplishing this step. In most cases, the process requirements can be met by more than one solvent, and the only satisfactory approach is an economic evaluation, which may involve the complete but preliminary design and cost estimate for more than one alternative. The ideal absorbent should:
· — have a high solubility for the solute(s) to minimize the need for absorbent;
· — have a low volatility to reduce the loss of absorbent and facilitate separation of solute(s);
· — be stable to maximize absorbent life and reduce absorbent makeup requirement;
· — be noncorrosive to permit use of common materials of construction;
· — have a low viscosity to provide low pressure drop and high mass and heat transfer rates;
· — be nonfoaming when contacted with the gas;
· — be nontoxic and nonflammable to facilitate its safe use;
· — be available, if possible within the process, or be inexpensive.
The most widely used absorbents are water, hydrocarbon oils, and aqueous solutions of acids and bases. For stripping, the most common agents are water vapor, air, inert gases, and hydrocarbon gases. Once an absorbent is selected, the design of the absorber requires the determination of basic physical property data such as density, viscosity, surface tension, and heat capacity. The fundamental physical principles underlying the process of gas absorption are the solubility and heat of solution of the absorbed gas and the rate of mass transfer. Information on both must be available when sizing equipment for a given application. In addition to the fundamental design concepts based on solubility and mass transfer, many practical details have to be considered during actual plant design
The second step is the selection of the operating conditions and the type of contactor. In general, operating pressure should be high and temperature low for an absorber, in order to minimize stage requirements and/or absorbent flow rate. In contrast, operating pressure should be low and temperature high for a stripper, in order to minimize stage requirements or stripping agent flow rate. However, because maintenance of a vacuum is expensive, strippers are commonly operated at a pressure just above ambient. A high temperature can be used, but it should not be so high as to cause undesirable chemical reactions. Choice of the contactor may be done on the basis of system requirements and experience factors such as those discussed in Sect. 8.5. Following these decisions it is necessary to calculate material and heat balance calculations around the contactor, define the mass transfer requirements, determine the height of packing or number of trays, and calculate contactor size to accommodate the liquid and gas flow rates with the selected column internals.
8.3.1 Gas solubilities
The most important physical property data required for the design of absorbers and strippers are gas-liquid equilibria. Since equilibrium represents the limiting condition for any gas-liquid contact, such data are needed to define the maximum gas purity and rich solution concentration attainable in absorbers, and the maximum lean solution purity attainable in strippers. Equilibrium data are also needed to establish the mass transfer driving force, which can be defined simply as the difference between the actual and equilibrium conditions at any point in a contactor.
At equilibrium, a component of a gas in contact with a liquid has identical fugacities in both the gas and liquid phase. For ideal solutions Raoult’s law applies:
![]()
(8.1)
where yA is the mole fraction of A in the gas phase, Ptot is the total pressure, ![]() is the vapor pressure of pure A, and xA is the mole fraction of A in the liquid. For nonideal mixtures Raoults law modifies into
is the vapor pressure of pure A, and xA is the mole fraction of A in the liquid. For nonideal mixtures Raoults law modifies into
![]()
(8.2)
where ![]() is the activity coefficient of solute A in the absorbent at infinite dilution. A more general way of expressing solubilities is through the vapor-liquid equilibrium constant K defined by
is the activity coefficient of solute A in the absorbent at infinite dilution. A more general way of expressing solubilities is through the vapor-liquid equilibrium constant K defined by
![]()
(8.3)
The value of the equilibrium constant K is widely employed to represent hydrocarbon vapor-liquid equilibria in absorption and distillation calculations. Correlations and experimental information for the equilibrium K values of hydrocarbons are available from various sources.
For moderately soluble gases with relatively little interaction between the gas and liquid molecules equilibrium data are usually represented by Henry’s law:
![]()
(8.4)
where pA is the partial pressure of component A in the gas phase. H is Henry’s constant, which has the units of pressure per composition. Usually H is dependent upon temperature, but relatively independent of pressure at moderate levels. In general, for moderate temperatures, gas solubilities decrease with an increase in temperature. Henry’s constants for many gases and solvents are tabulated in various literature sources. Examples of Henry’s constants for a number of gases in pure water are given in Fig. 8.2.
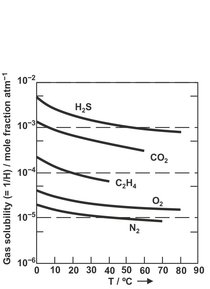
Fig. 8.2: Solubilities of various gases in water expressed as the reciprocal of the Henry’s law constant. Adapted from [7].
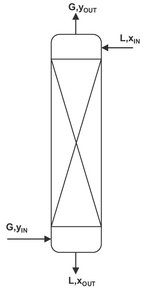
Fig. 8.3: Continuous steady-state operation in a counter-current absorber.
8.3.2 Minimum absorbent flow
For each feed gas flow rate, absorbent composition, extent of solute absorption, operating pressure and operating temperature, a minimum absorbent flow rate exists that corresponds to an infinite number of countercurrent equilibrium contacts between the gas and liquid phases. As a result a tradeoff exists in every design problem between the number of equilibrium stages and the absorbent flow rates at rates greater than the minimum value. This minimum absorbent flow rate LMIN is obtained from a mass balance over the whole absorber, assuming equilibrium is obtained between in incoming gas and outgoing absorbent liquid in the bottom of the column. An overall mass balance over the column illustrated by Fig. 8.3 gives the following result:
![]()
(8.5)
Introduction of the assumption that the outgoing liquid is in equilibrium with in incoming gas (χOUT = KYIN) gives the minimum absorbent flow:
![]()
(8.6)
A similar derivation of the minimum stripping gas flow rate GMIN for a stripper results in an analogous expression:
![]()
(8.7)
8.3.3 Number of equilibrium stages
If the gas and liquid flow rates in absorbers and strippers are relatively constant and temperature effects can be neglected, the number of equilibrium stages N may be calculated with the Kremser method:
![]()
(8.8)
and
![]()
(8.9)
where the solute absorption factor A and stripping factor S are given by
![]()
Values of L and G in moles per unit time may be taken as the entering values. Values of Ki depend mainly on temperature, pressure and liquid-phase composition.