Process Technology: An Introduction - Haan A.B. 2015
9 Adsorption and ion exchange
9.2 Adsorption fundamentals
9.2.1 Industrial adsorbents
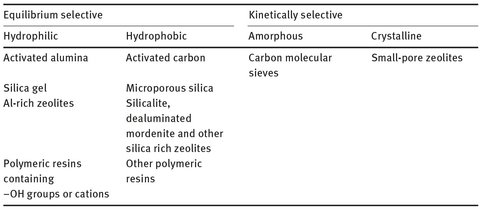
The role of the adsorbent is to provide the selectivity and capacity required for the separation of a mixture. In the majority of adsorptive separation processes the selectivity is provided by the physical adsorption equilibrium. Because the adsorption forces depend on the nature of the adsorbing molecule as well as on the nature of the surface, different substances are adsorbed with different affinities. In Tab. 9.2 a simple classification scheme is given where equilibrium-controlled adsorbents are primarily divided into hydrophilic and hydrophobic surfaces. It is clear that adsorbents with hydrophilic surfaces will preferentially attract polar molecules, in particular water, and adsorbents with hydrophobic surfaces nonpolar molecules.
Tab. 9.1: Examples of industrial adsorptive and ion exchange separations.
Separation |
Application |
Adsorbent |
|
Gas bulk separations |
Normal paraffins, isoparaffins, aromatics |
Zeolite |
N2/O2 |
Zeolite |
|
O2/N2 |
Carbon molecular sieve |
|
CO, CH4, CO2, N2, NH3/H2 |
Zeolite, activated carbon |
|
Hydrocarbons from vent streams |
Activated carbon |
|
|
Gas purification |
H2O removal from cracking gas, natural gas, air etc. |
Silica, alumina, zeolite |
CO2 from C2H4, natural gas etc. |
Zeolite |
|
Organics from vent streams |
Activated carbon, silicalite |
|
Sulfur compounds from organics |
Zeolite |
|
Solvents and odors from air |
Activated carbon, silicalite |
|
|
Liquid bulk separations |
Normal paraffins, isoparaffins, aromatics |
Zeolite |
p-Xylene/o-xylene, m-xylene |
Zeolite |
|
Fructose/glucose |
Zeolite, ion exchange resins |
|
|
Liquid purifications |
H2O removal from organic solvents |
Silica, alumina, zeolite |
Organics from water |
Activated carbon, silicalite |
|
Odor and taste components from drinking water |
Activated carbon |
|
Product decolorizing |
Activated carbon |
|
Fermentation product recovery |
Activated carbon, affinity agents |
|
|
Ion exchange |
Water softening |
Polymeric ion exchange resins |
Water demineralization |
||
Water dealkalization |
||
Decolorization of sugar solutions |
||
Recovery of uranium from leach solutions |
||
Recovery of vitamins from fermentation broths |
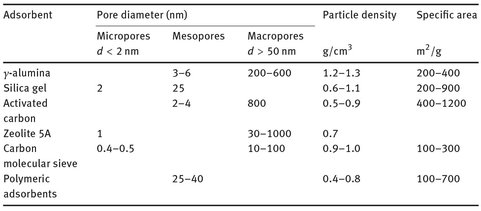
Besides a high selectivity, a high capacity is also desirable since the adsorption capacity determines the size and therefore the cost of the adsorbent bed. To achieve a high adsorptive capacity commercial adsorbents are made from microporous materials with a high specific area. The most important properties of various microporous industrial adsorbents are listed in Tab. 9.3. According to the definition of the IUPAC, the pores in adsorbents fall into three categories:
· (1) micropores: 2 nm;
· (2) mesopores: 2-50 nm;
· (3) macropores: > 50 nm.
Tab. 9.2: Classification of commercial adsorbents.
In a micropore the guest molecule never escapes from the force field of the solid surface, while in mesopores and macropores the molecules in the central region are essentially free from the surface force field. Macropores provide very little surface area relative to the pore volume and hardly contribute to the adsorptive capacity. Their main role is to provide a network of superhighways to facilitate rapid penetration of molecules into the interior of the adsorbent particle.
In industrial processes only four types of adsorbents dominate in usage: activated carbon, silica gel, activated alumina, and molecular sieve zeolites. Among these four types, activated carbon is most often used for removing hydrophobic organic species from both gas and aqueous liquid streams. Activated carbon is produced in many different forms, which differ mainly in pore size distribution and surface polarity. For liquid-phase adsorption a relatively large pore size is required, while the activated carbons used in gas adsorption have much smaller pores. Commercial carbons are used in the form of hard pellets, granules, cylinders, spheres, flakes, or powders. Silica gels represent an intermediate between highly hydrophilic and highly hydrophobic surfaces. Most often these adsorbents are used for removing water from various gases, but hydrocarbon separations are also sometimes feasible. Activated alumina is essentially a microporous (amorphous) form of Al2O3, which has quite a high affinity for water and is often used in drying applications for various gases. Like with silica gels, the water bond with the alumina surface is not as strong as with zeolites, so that regeneration of aluminas can often be accomplished at somewhat milder temperatures. As can be seen in Fig. 9.2, activated alumina can have a higher ultimate water capacity, but the zeolites have a higher capacity at low water partial pressures. Thus zeolites are typically chosen when very high water removal is necessary, while activated alumina is preferred if adsorbent capacity is more important.
Tab. 9.3: Properties of industrial adsorbents.
Polymer-based adsorbents are presently used in a few operations removing organic constituents from vent gas streams, such as the removal of acetone from air. As illustrated in Fig. 9.3, these materials are usually styrene-divinylbenzene copolymers, which in some cases have been derivatized to give the desired adsorption properties.
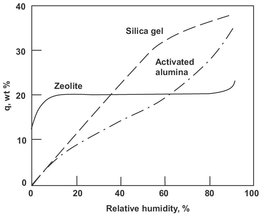
Fig. 9.2: Comparison of water adsorption on various adsorbents.
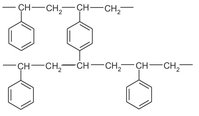
Fig. 9.3: Structure of styrene-divinylbenzene copolymer adsorbents.
In addition to equilibrium adsorption, selectivity of adsorbents may also originate from two important other separating mechanisms. Exclusion of certain molecules in the feed because they are too large to fit into the pores of the adsorbent (molecular sieving) and differences in the diffusion of different adsorbing species in the pores of the adsorbent (kinetic effect). Significant kinetic selectivity is a characteristic feature of molecular sieve adsorbents such as carbon molecular sieves and zeolites. Molecular sieve zeolites are highly crystalline aluminosilicate structures with highly regular channels and cages, as displayed in Fig. 9.4. Zeolites are selective for polar hydrophilic species, and very strong bonds are created with water, carbon dioxide, and hydrogen sulfide, while weaker bonds are formed with organic species. Molecular sieving is possible when a channel size is of molecular dimensions, restricting the diffusion sterically. As a result small differences in molecular size or shape can lead to very large differences in diffusivity. In the extreme certain molecules or a whole class of compounds may be completely excluded from the micropores. This is illustrated in Fig. 9.5, where a range of molecular sizes is compared with the channel diameters of various zeolites. The most important examples of such a processes are the separation of linear hydrocarbons from their branched and cyclic isomers using a 5A zeolite adsorbent and air separation over carbon molecular sieve or 4A zeolite, in which oxygen, the faster diffusing component, is preferentially adsorbed.
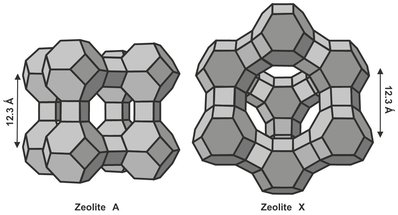
Fig. 9.4: Schematic diagrams of structures of two common molecular sieve zeolites.
9.2.2 Adsorption equilibrium
In adsorption a dynamic equilibrium is established for the distribution of the solute between the fluid and the solid surface. This equilibrium is commonly expressed in the form of an isotherm, which is a diagram showing the variation of the equilibrium adsorbed-phase concentration or loading with the fluid-phase concentration or partial pressure at a fixed temperature. For pure gases, experimental physical adsorption isotherms have shapes that are classified into five types by Brunauer, as shown in Fig. 9.6. Both types I and II are desirable isotherms, exhibiting strong adsorption. When the amount adsorbed is low, the isotherm should approaches a linear form and the following form of Henry’s law, called the linear isotherm, is obeyed:
![]()
(9.2)
where q is the equilibrium loading or amount adsorbed per unit mass of adsorbent, p is the partial pressure of a gas, and c is the concentration of the solute. b is a temperaturedependent empirical constant related to the heat of adsorption through an Arrheniustype equation:
![]()
(9.3)
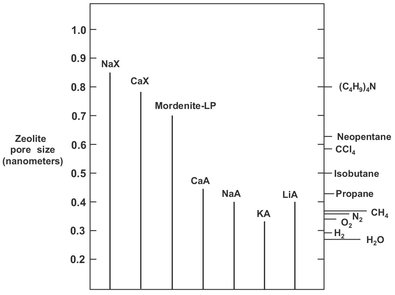
Fig. 9.5: Comparison of molecular dimensions and zeolite pore sizes.
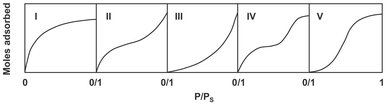
Fig. 9.6: The Brunauer classification of adsorption isotherms.
For an exothermic process, Δ Hads is negative, and the Henry constant decreases with increasing temperature. At higher concentrations, competition for surface sites and significant interaction between adsorbed molecules begin to play a role. Under these conditions many industrial systems exhibit the type I behavior, which is commonly represented by the ideal Langmuir model:
![]()
(9.4)
In the Langmuir model, qs is the saturation limit and b is again an equilibrium constant directly related to the Henry constant. This expression is the correct form to represent a type I isotherm, since at low pressure it approaches Henry’s law, while at high pressure it tends asymptotically to the saturation limit. Although only few systems behave exactly to the Langmuir model, it provides a simple qualitative representation of the behavior of many systems, and it is therefore widely used. The Langmuir model can also be simply extended to multicomponent systems, reflecting the competition between species for the adsorption sites:
![]()
(9.5)
In equilibrium-based separations the selectivity of the adsorbent is determined by the separation factor α’, which for the multicomponent Langmuir model simply corresponds to the ratio of the equilibrium constants:
![]()
(9.6)
Because this selectivity is independent of composition, the ideal Langmuir model is often referred to as the constant separation factor model.
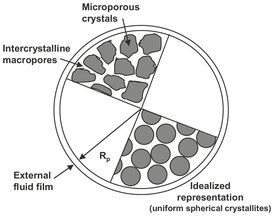
Fig. 9.7: Resistances to mass transfer in a composite adsorbent pellet. Adapted from [102].
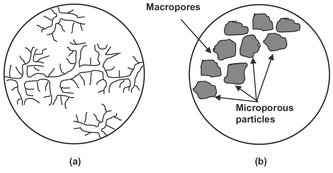
Fig. 9.8: Homogeneous and composite microporous adsorbent particle. Adapted from [102].
9.2.3 Adsorption kinetics
As illustrated in Fig. 9.7, the adsorption of a solute onto the porous surface of an adsorbent requires the following steps:
· (1) external mass transfer of the solute from the bulk fluid through a thin film or boundary layer, to the outer solid surface of the adsorbent;
· (2) internal mass transfer of the solute by pore diffusion from the outer surface of the adsorbent to the inner surface of the internal pore structure;
· (3) surface diffusion along the porous surface;
· (4) adsorption of the solute onto the porous surface.
During regeneration of the adsorbent, the reverse of the four steps occurs. In general the rate of physical adsorption is controlled by diffusional limitations rather than by the actual rate of equilibration at a surface. A laminar fluid film or boundary layer through which the solute must diffuse always surrounds the outside of an immersed particle. This rate of mass transfer is normally expressed in terms of a simple linear rate expression:
![]()
(9.7)
However, under most practical conditions the external film resistance is seldom rate limiting, so that internal mass transfer generally controls the adsorption/desorption rate. From this perspective adsorbents may be divided into two broad classes: homogeneous and composite. Fig. 9.8 illustrates that in homogeneous adsorbents (activated alumina, silica gel) the pore structure persists throughout the particle, while the composite adsorbent particles (zeolites, carbon molecular sieves) are formed by aggregation of small microporous microparticles. As a result the pore-size distribution in composite particles has a well-defined bimodal character, with micropores within the microparticles connected through macropores within the pellet. Transport in a macropore can occur by bulk molecular diffusion, Knudsen diffusion, surface diffusion, and Poiseuille flow. In liquid systems bulk molecular diffusion is generally dominant, but in the vapor phase the contributions from Knudsen and surface diffusion may be large or even dominant. Knudsen diffusion becomes dominant when collisions with the pore wall occur more frequently than collisions with diffusing molecules. In micropores the diffusing molecule never escapes from the force field of the pore wall, and the Knudsen mechanism no longer applies. Diffusion occurs by jumps from site to site, just as in surface diffusion, and the diffusivity becomes strongly dependent on both temperature and concentration. The selectivity in a kinetically controlled adsorption process depends on both kinetic and equilibrium effects. This kinetic selectivity can be approximated when two species (A and B) diffuse independently and their isotherms are also independent. Under these conditions the ratio of their uptakes at any time will be given by
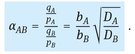
(9.8)
A typical example is shown in Fig. 9.9, where the equilibrium adsorption and loading curves of oxygen and nitrogen on carbon molecular sieves is shown. The dramatic difference between the uptake rates is caused by the fact that the pore diameter of the carbon is just very slightly larger than the diameters of the two gases. As a result nitrogen has much more difficulty traversing a pore than oxygen. Thus, even though there is virtually no equilibrium selectivity, the operation in the kinetic region results in very high selectivities for oxygen/nitrogen separation.
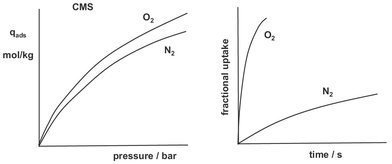
Fig. 9.9: Equilibrium adsorption and uptake rates for carbon molecular sieves used in air separation.
9.2.4 Fixed-bed adsorption
In a fixed bed a nearly solute-free liquid or gas effluent can be obtained until the adsorbent in the bed approaches saturation. Under the conditions that external and internal mass-transfer resistances are very small, plug flow is achieved, axial dispersion is negligible, the adsorbent is initially free of adsorbate, and the adsorption isotherm begins at the origin where equilibrium between the fluid and the adsorbent is instantaneously achieved. Under these ideal fixed-bed adsorption conditions the instantaneous equilibrium results in a shock like wave, the stoichiometric front (Fig. 9.10), moving as a sharp concentration step through the bed. Upstream of the front the adsorbent is saturated with adsorbate, and the concentration of solute in the fluid is that of the feed. In the upstream region the adsorbent is spent. Downstream of the stoichiometric front and in the exit fluid, the concentration of the solute in the fluid is zero, and the adsorbent is still adsorbate free. After a period of time, called the stoichiometric time, the stoichiometric wave front reaches the end of the bed, and the concentration of the solute in the fluid rises abruptly to the inlet value. Because no further adsorption is possible, this point is referred to as the breakthrough point.
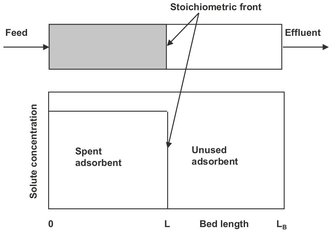
Fig. 9.10: Stoichiometric equilibrium concentration front for ideal fixed-bed adsorption.
For ideal fixed-bed adsorption, the location of the concentration wave front L as a function of time can be obtained from a material balance under adsorption equilibrium considerations. The adsorbent loading in equilibrium with the feed is designated by the adsorption isotherm. A material balance over the adsorbate gives the position of the front in the adsorbent bed:
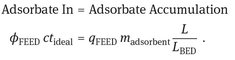
(9.9)
In real fixed-bed adsorption, internal and external transport resistance are finite, and axial dispersion can be significant. These factors contribute to the development of broader S-shaped concentration fronts like those in Fig. 9.11. The S-shaped curve is called the breakthrough curve. The steepness of the breakthrough curve determines the extent to which the capacity of an adsorbent bed can be utilized. Another complication is the fact that the steepness of the concentration profiles increases or decreases with time, depending on the shape of the adsorption isotherm. If the adsorption isotherm is curved, regions of the front at a higher concentration move at a velocity different from regions at a lower concentration. Thus, for a linear isotherm the shape remains constant. For a favorable isotherm of the Langmuir type, highconcentration regions move faster than low-concentration regions, and the wave front steepens with time until a constant pattern front is developed.
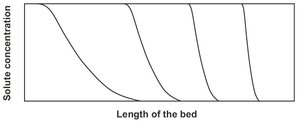
Fig. 9.11: Self-sharpening wave front caused by a favorable adsorption isotherm.