Process Technology: An Introduction - Haan A.B. 2015
12 Membrane separations
12.5 Electrodialysis
The principle of electrodialysis is depicted in Fig. 12.22. In this process an electric potential gradient is used to remove ions from an aqueous solution with charged ionselective membranes. A large number of anion— and cation-exchange membranes are placed in an alternating pattern between a cathode and an anode in a plate-and-frame stack. The positively charged sodium ions migrate to the cathode but are unable to pass the positively charged membranes. In the same manner the negatively charged sulfate ions migrate to the anode but cannot pass the negatively charged membranes. The resulting overall effect is that every second cell becomes depleted of salt, while the adjacent cells become concentrated in salt. The degree of concentration is determined by the flow rate of the feed solution through the stack.
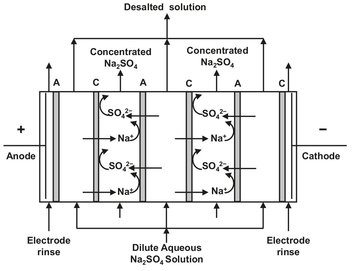
Fig. 12.22: Plate-and-frame electrodialysis stack.
Electrodialysis is used widely to desalinate brackish water. In desalination one of the most attractive features of electrodialysis is its energy efficiency compared to evaporation or reverse osmosis. Because the electric current needed to desalinate a solution is directly proportional to the quantity of ions transported through the membrane, electrodialysis is almost always the lowest-cost process in the 500 to 2000 ppm range. A very special reverse application of desalination is the production of a salt concentrate from seawater in Japan. The process is also used in the food industry to deionize cheese whey, and to deacidifiy wine and fruit juices. A number of other applications exist in wastewater treatment, particularly the regeneration of waste acids used in metal pickling operations and the removal of heavy metals from electroplating rinse waters. A very recent industrial application is the removal of organic acids from a fermentation broth.
Bipolar membranes consist of an anionic and cationic membrane laminated together. At the interface between the two membranes ions are generated by the watersplitting reaction. The liberated hydrogen ions migrate to the cathode, while the hydroxyl ions migrate to the anode. When used in an electrodialysis process, bipolar membranes can divide a neutral salt into the conjugate acid and base. Unfortunately the process is limited to the generation of relatively dilute acid and base solutions.
Nomenclature
A |
proportionality constant |
|
C |
concentration |
[mol m-3] |
D |
coefficient of diffusion |
[m2 s-1] |
F |
feed flow |
[mol s-1] |
Hi |
henry coefficient of component i |
[mol bar-1] |
J |
component flux |
[mol s-1 m-2] |
k |
proportionality constant |
[kg s-1 m-2 bar-1 mol s-1 m-2 v-1] |
K¡ |
distribution coefficient of component i |
[-] |
N |
permeation rate |
[kg s-1 m-2, mol s-1 m-2] |
P |
pressure |
[Pa, bar] |
P |
permeate flow |
[mol s-1] |
Pm |
membrane permeability |
|
PM,i |
permeability of component i |
|
R |
retentate flow |
[mol s-1] |
Ri |
retention of i |
[-] |
Rtot |
membrane resistance |
|
S |
solubility |
[mol m-3] |
SF |
separation factor |
[-] |
x, y |
mole fractions |
[-] |
δM |
membrane thickness |
[m] |
ΔX |
driving force |
[Pa, bar, V] |
η |
viscosity |
[Pa · s] |
Π |
osmotic pressure |
[Pa, bar] |