Process Technology: An Introduction - Haan A.B. 2015
13 Crystallization and precipitation
13.1 Introduction
Crystallization is one of the oldest unit operations in the portfolio of industrial separations. Large quantities of crystalline products, such as sodium chloride, sucrose, and fertilizer chemicals (ammonium nitrate, ammonium phosphates, urea) are manufactured commercially. In the production of organic chemicals, crystallization is used to recover a product, to refine intermediate chemicals, and to remove undesired salts. Crystalline products coming from the pharmaceutical, fine chemical and dye industries are produced in relatively small quantities, but represent a valuable and important industrial sector.
Crystallization distinguishes itself from other separation technologies in that a solid phase is generated. In the process the feed consists of a homogeneous solution which is made supersaturated by cooling and/or evaporation of the solvent to obtain the desired solute in a solid form. The process of solid-phase formation is termed crystallization, and the operation occurs in a vessel called a crystallizer. Functions which can be achieved by crystallization include separation, purification, concentration, solidification, and analysis. Crystallization usually occurs on added seed crystals or on very small crystals, nucleii, broken off of other crystals. The crystal particles grow and become larger until they are removed (harvested) from the crystallizer. Although the crystals are often of high purity, their performance is also characterized by their inherent shape and size distribution. These solid-phase properties are especially important, because crystallization is frequently the initial step in a solid processing sequence, similar to that shown in Fig. 13.1. After crystallization the solids are normally separated from the crystallizer liquid, washed, and discharged to downstream drying equipment. Since product size and the concentration of suspended solids are controlled to a large extent in the crystallizer, predictable and reliable crystallizer performance is essential for smooth operation of the downstream system.
The main advantages of crystallization are that nearly pure solid product can be recovered in the desired shape in one separation stage. With care in design, product purity in excess of 99.0 % can be attained in a single crystallization, separation, and washing sequence. This large separation factor is one of the reasons that make crystallization a desirable separation method. A second reason why crystallization is often the separation method of choice is that it can produce uniform crystals of the desired shape. During crystallization the conditions are controlled so that the crystals have the desired physical form for direct packaging and sale. This is important when a solid product is desired. The major disadvantages of crystallization are that purification of more than one component and/or full solute recovery are not attainable in one stage. Thus, additional equipment is required to remove the solute completely from the remaining crystallizer solution. Since crystallization involves processing and handling of a solid phase, the operation is normally applied when no alternative separation technique is discernable. Some important considerations that may favor the choice of crystallization as the preferred option over other separation techniques such as distillation include the following.
· — Solute is heat sensitive and/or a high boiler that decomposes at the high temperatures required to conduct distillation.
· — Low relative volatility between the solute and contaminants and/or the existence of azeotropes between solute and contaminants.
· — Solid solute product. For example, after purification via distillation a solute must be solidified by flaking or prilling and crystallization may be more convenient to employ.
· — Comparative economics favor crystallization. If distillation requires high temperatures and energy usage, crystallization may offer economic incentives.
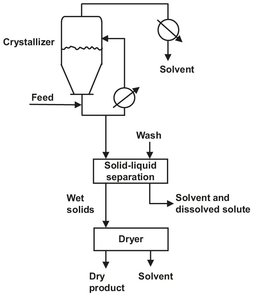
Fig. 13.1: Schematic of a crystallization initiated solids processing sequence.
Precipitation is an important separation method in the production of many fine chemicals and in mineral processing. It is a related process since solutes dissolved in a solvent precipitate out. However, the precipitate is usually amorphous and will have a poorly defined shape and size. Precipitates are often aggregates of several species and may include salts or occluded solvent. Thus precipitation serves as a “rough cut” to either remove impurities or to concentrate and partially purify the product.