Process Technology: An Introduction - Haan A.B. 2015
13 Crystallization and precipitation
13.4 Crystallization from solutions
13.4.1 Basic operations
The basic functions a system for the crystallization from solutions should provide are:
· (1) a means of generating supersaturation which may include provision for addition or removal of heat;
· (2) a vessel to provide sufficient residence time for the process streams to approach equilibrium and the crystals to grow to a desired size;
· (3) mixing regime to provide an environment for uniform crystal growth;
· (4) possibly a capability of selectively removing fines or coarse product to control the crystal size distribution.
Solution crystallizers are generally classified into one of five categories according to the method by which supersaturation is achieved. The technique employed to generate supersaturation in a solution is referred to as the mode of operation. The mode chosen by the designer is strongly influenced by the phase-equilibrium characteristics of the system, and it dictates the material and energy balance requirements of the system. The common techniques for producing solids from a solution include:
· — lowering the temperature of the feed solution by direct or indirect cooling. If solute solubility is strongly temperature dependent, this is the preferred approach;
· — adding heat to the system to remove solvent and thus “salt out” the solute; this technique is effective if solubility is insensitive to temperature;
· — vacuum cooling the feed solution without external heating; if solubility is strongly dependent on temperature this method is attractive;
· — combining techniques; especially common is vacuum cooling supplemented by external heating for systems whose solubility has an intermediate dependence on temperature;
· — adding a nonsolvent; this is a common technique for precipitating solute from solution and is useful as both a laboratory technique and as an industrial process for product recovery.
These methods can be employed in single— or multistage crystallization or in batch operations. Generally, production rates over 50 tons/day justify continuous operation. The continuous operations tend to have higher yield and require less energy than batch, but batch is more versatile. Multistage operation is employed where evaporative requirements exceed the capabilities of a single vessel and/or energy costs dictate staging of the operation. Another reason for staging may sometimes be the production of more uniform and/or larger crystals. Operation of crystallizers in series generates crystal-size distributions having narrower size spread than the same volume of crystallizers in parallel. Batch crystallizers produce a narrower CSD than continuous well-mixed units. Although numerous design methods and kinetic theories exist to analyze specific crystallizer configurations, no clear-cut guidance is available for the choice between crystallizer types and/or modes of operation.
13.4.2 Cooling crystallizers
Cooling crystallizers obtain supersaturation by cooling the hot saturated solution. They are very desirable when they work because of their high yield and low energy consumption. Cooling crystallization is applicable to systems where temperature has a large influence on solubility (e.g. ammonium alum and soda). This method is not useful for systems like NaCl where temperature has little effect on solubility. When cooling is the selected mode by which supersaturation is generated, heat can be transferred through an external cooling surface or through coils or a jacket internal to the crystallizer body. The forced circulation crystallizer is a simple highly effective unit designed to provide high heat-transfer coefficients in either an evaporative or cooling mode. A schematic diagram in which slurry is withdrawn from the crystallizer body and pumped through an external heat exchanger is shown in Fig. 13.8. Most systems are operated with a high recirculation rate to provide good mixing inside the crystallizer and high rates of heat transfer to minimize encrustation. The tendency of the solute to form encrustations on the cooling surface often limits their operation by restricting the temperature of the cooling liquid and the temperature decrease of the slurry flowing through the heat exchanger. High heat transfer rates reduce the formation of encrustations considerably by minimizing the temperature difference over the heat transfer surface. It is not uncommon to limit the decrease in magma temperature to about 3-5 °C. The feed is commonly introduced into the circulation loop to provide rapid mixing with the magma (mixture of crystals and solution) and minimize the occurrence of regions of high supersaturation, which can lead to excessive nucleation.
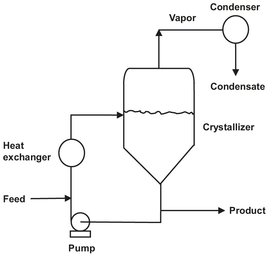
Fig. 13.8: Schematic of forced circulation crystallizer with external cooling.
The use of a conventional heat exchanger can be avoided by employing direct-contact cooling (DCC) in which the product liquor is allowed to come into contact with a cold heat-transfer medium. Other potential advantages include better heat transfer and smaller cooling load. However, problems include product contamination from the coolant and the cost of extra processing required to recover the coolant for further use. Crystallization processes employing DCC have been used successfully in recent year for dewaxing of lubricating oils, desalination of water and production of inorganic salts from aqueous solutions.
13.4.3 Evaporating and vacuum crystallizers
Evaporative crystallizers supersaturate the solution by removing solvent through evaporation. These crystallizers are used when temperature has little effect on solubility (e.g. NaCl) or with inverted solubilities (e.g. calcium acetate). The crystallizer may be as simple as a shallow open pan heated by an open fire. Steam-heated evaporators are widely used to produce common salt from brine and sugar refining. These applications often use an evaporative crystallizer-containing calandria (steam chest). The magma circulates by dropping through the central downcomer and then rises as it is heated in the calandria. At the top some of the solution evaporates, increasing the supersaturation causing crystal growth. Many types of forced circulation evaporating crystallizers are also used on a large-scale. These systems are similar to the one shown in Fig. 13.8 and obviously very similar to evaporators without crystallization. Operational problems with evaporative crystallizers can be caused by scale formation on the heat exchanger surfaces or at the vapor-liquid interface in the crystallizer. Such problems can be overcome by not allowing vaporization or excessive temperatures within the exchanger and by proper introduction of the circulating magma into the crystallizer. The latter may be accomplished by introducing the magma below the surface of the magma in the crystallizer.
The evaporators are often connected together to make a multiple-effect cascade, as shown in Fig. 13.9. Here the vapor from the first unit is used as the steam to heat the second unit. This reduces the steam requirements per kg of product. The pressure must be varied as shown in the figure, so that the condensing vapor will be at a temperature greater than the boiling temperature in the second stage. A variety of ways connecting the different stages have been developed.
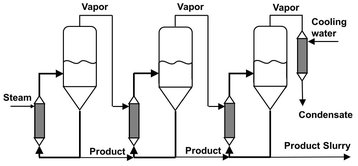
Fig. 13.9: Multiple-effect evaporating crystallizer cascade for ammonium sulfate.
Vacuum crystallizers utilize evaporation to both concentrate the solution and cool the mixture. They combine the operating principles of evaporative and cooling crystallizers. The hot liquid feed enters the crystallizer at a higher pressure and temperature, where it flashes and part of the feed evaporates. This flashing causes adiabatic cooling of the liquid. Crystals and mother liquor exit the bottom of the crystallizer. Because of the vacuum equipment required to operate at pressures of 5-20 mbar, the vacuum crystallizers are considerably more complex than other types of crystallizers and most common in large systems.
13.4.4 Continuous crystallizers
Many different continuously operated crystallizers are available. The majority can be divided into three basic types: forced circulation, fluidized bed (OSLO), and draft-tube agitated units. A forced-circulation crystallizer has already been discussed and is shown in Fig. 13.8. Fig. 13.10 shows a draft-tube-baffle (DTB) crystallizer designed to provide preferential removal of both fines and classified product. A relatively low speed propeller agitator is located in a draft tube, which extends to a few inches below the liquor level in the crystallizer. The steady movement of magma up to the surface produces a gentle uniform boiling action over the whole cross-sectional area of the crystallizer. Agitation effects are absent between the baffle and the outside wall of the crystallizer. This provides a settling zone that permits regulation of the magma density and control of the removal of excess nuclei. Flow through the annular zone can be adjusted to remove only crystals below a certain size and dissolve them in the fines dissolution exchanger. Feed is introduced to the fines circulation line to dissolve nuclei resulting from feed introduction.
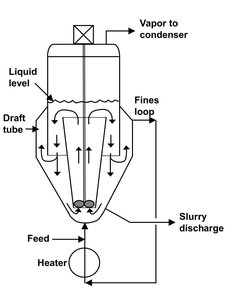
Fig. 13.10: Schematic of a draft-tube-baffled (DTB)
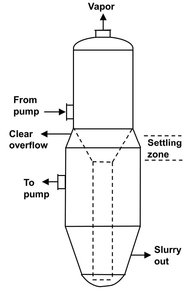
Fig. 13.11: Oslo crystallizer. crystallizer.
Another type of continuous crystallizer is the Oslo fluidized-bed crystallizer shown in Fig. 13.11. In units of this type a bed of crystals is suspended in the vessel by the upward flow of supersaturated liquor in the annular region surrounding a central downcomer. The objective is to form a supersaturated solution in the upper chamber and then relieve the supersaturation through growth in the lower chamber. The use of the down flow pipe in the crystallizer provides good mixing in the growth chamber.