Process Technology: An Introduction - Haan A.B. 2015
13 Crystallization and precipitation
13.7 Melt crystallization
In some crystallization systems the use of a solvent can be avoided. Melt crystallization is the process where crystalline material is separated from a melt of the crystallizing species by direct or indirect cooling, until crystals are formed in the liquid phase. Two basic techniques of melt crystallization are gradual deposition of a crystalline layer on a chilled surface or fast crystallization of a discrete crystal suspension in an agitated vessel. Zone melting relies on the distribution of solute between the liquid and solid phases to effect a separation. Normal freezing is the slow solidification of a melt. The impurity is rejected into the liquid phase by the advancing solid interface. The basic requirement of melt crystallization is that the composition of the crystallized solid differs from that of the liquid mixture from which it is deposited. The process is usually operated near the pure-component freezing temperature. High or ultrahigh product purity is desired in many of the melt purification processes. Single-stage crystallization is often not sufficient to achieve the required purity of the final product. Further separation can be achieved by repeating the crystallization step or by countercurrent contacting of the crystals with a relatively pure liquid stream.
Since there is no solvent, melt crystallization has the advantage that no solvent removal and recovery are required, and that contamination by the solvent is impossible. However, there is also no way to influence the melt properties (viscosity, diffusivity), and the chemicals being purified must be stable at the melting point. For salts with very high melting points, solution crystallization is less expensive. At the lower temperatures possible in solution, crystallization distribution coefficients can be orders of magnitude more favorable. These comparisons show why crystallization from solution is much more common for bulk separations. Melt crystallization becomes advantageous when the presence of a solvent would be detrimental, or when highly pure products are desired. Currently the interest in wider application of melt crystallization is stimulated by the energy-saving potential in large scale processing, because solvent evaporation is absent, and the heat of fusion is several times less than the heat of vaporization.
Multiple countercurrent contacting stages are possible by conducting melt crystallization inside a column in a manner somewhat analogous to distillation. The main incentive is to attain higher purity product than that which can be achieved in a single stage of conventional crystallization. The concept of a column crystallizer is to form a crystal suspension and to force the solids to flow counter currently against a stream of enriched reflux liquid by gravity or rotating blades. At the end of the crystallizer the crystals are melted. A portion of the melt is removed as product and the remainder is returned to the system as enriched reflux to wash the product crystals. One of the early column crystallizers, shown schematically in Fig. 13.16, was developed for the separation of xylene isomers. In this unit p-xylene crystals are formed in a scraped-surface chiller above the column and fed to the column. The crystals move countercurrently downward to impure liquid in the upper portion of the column and melted p-xylene in the lower part of the column. Impure liquor is withdrawn from an appropriate point near the top of the column of crystals, while pure product, p-xylene, is removed from the bottom of the column. An inherent limitation of column crystallization is the difficulty of controlling the solid-phase movement, because the similar phase densities make gravitational separation difficult.
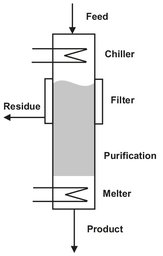
Fig. 13.16: Vertical column melt crystallizer.
The Sulzer MWB system, schematically depicted in Fig. 13.17, is an example of a commercial melt crystallization process that uses the gradual deposition of solids on a chilled surface. Crystal growth is on the inside of a battery of tubes through which melt is flowing. During crystallization the front of crystals advances into the direction of the mother liquor. This buildup of a solids layer requires sequential operation. Steps include partial freezing of a falling film of melt inside vertical tubes, followed by slight heating, a “sweating” operation, and complete melting and recovery of the refined product. The recovered product melt can be put through the cycle again to increase purity or fresh feed can be introduced to the cycle. The process has been used on a large scale in the purification of a wide range of organic substances.
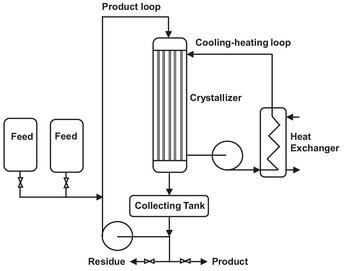
Fig. 13.17: Sulzer MWB melt crystallizer system.
Nomenclature
B0 |
homogeneous primary nucleation rate |
[nuclei/unit time · unit volume] |
c |
actual solution concentration |
[mol m-3] |
c* |
equilibrium saturation concentration |
[mol m-3] |
C |
crystal production rate |
[kg/unit time] |
cv |
coefficient of variation |
[-] |
ΔEG |
activation energy |
[J mol-1 K-1] |
FS |
mass feed rate of solvent, solute free |
[kg/unit time] |
G |
crystal growth rate |
[m/unit time] |
ΔHM |
enthalpy of melting |
[J mol-1] |
k |
rate constant |
|
L |
crystal size |
[m] |
LD |
(dominant) crystal size |
[m] |
n |
population density |
[number of crystals m-1 m-3] |
N |
number of crystals |
[number of crystals/unit volume] |
Q |
volumetric discharge rate |
[m3/unit time] |
R |
gas constant |
[8.314 J mol-1 K-1] |
RSE |
unit solvent evaporated per unit solvent fed |
[-] |
s |
relative super saturation |
[-] |
S |
solution removal rate |
[kg/unit time] |
SS |
mass discharge rate of solvent, solute free |
[kg/unit time] |
t |
time |
[s] |
T (TM) |
temperature (at melting point) |
[K] |
VS |
volume of Magma (suspension or clear liquor) |
[m3] |
XS |
solubility of solids, mole fraction |
[-] |
X |
mass of dissolved solute / mass solvent |
[ |
Y |
yield |
[-] |
γS |
activity coefficient of solvent |
[-] |
σ |
standard deviation of distribution |
[m] |