Process Technology: An Introduction - Haan A.B. 2015
18 Process safety
18.1 Safety problems in chemical plants
Large-scale chemical production is one of the most important industrial activities. Although its products play a key role in human nutrition, health, quality of life, and welfare, many people regard chemical production as dangerous. Despite the good safety records of chemical plants compared to other industries, grave accidents have occurred, endangering human life and the environment, and causing considerable damage. The names Flixborough (1974), Seveso (1976), Bhopal (1984), and Basel (1986) call dramatic events to mind. Three types of events are traditionally associated with the chemical industry:
· — Releases and spills (Seveso, Bhopal): An uncontrolled release from a closed facility requires an undesired opening of the containment or damage to the walls of vessels, process equipment, fittings, or piping. The release of liquids and solids generally poses a danger to the environment by penetrating into the soil. Gaseous substances are transported by the motion of ambient air and may spread over large areas.
· — Fires (Basel): Fires in chemical plants can occur if flammable substances are released and come in contact with an ignition source. The effects of fires relate to the action of heat and the release of pollutants.
· — Explosions (Flixborough): The best known is the instantaneous decomposition of sensitive chemical compounds to gaseous species. The second chemical explosion is the rapid oxidation of gases or finely dispersed particles in oxidizing gases. A highly exothermic reaction heats the gases to a high temperature so quickly that the pressure in confined systems increases dramatically. The third type of chemical explosion is the “thermal runaway” explosion, initiated by a homogeneous exothermic reaction that gets out of control. The accompanying temperature rise can lead to a dangerous pressure buildup by raising the vapor pressure of reactants or by initiating another reaction that liberates gases.
Damage caused by these events comes about through the direct action of chemicals on humans and the environment or by the indirect action of liberated energy. In the Bhopal accident many people died after inhaling a dangerous gas that had escaped from a tank storage facility. Most of the damage from the Basel warehouse fire was due to firefighting water carrying starting material, decomposition products, and combustion products out of the storage facility, harming the ecosystem of the Rhine. The consequences of the Flixborough explosion occurred largely through the destructive action of the pressure wave, initiated by ignition of a cloud of escaped flammable gases.
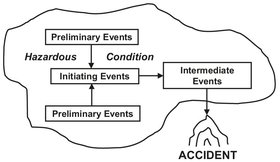
Fig. 18.1: Events that lead to an accident. Adapted from [196].
However, these accidents do not just happen. They are the result of a long process, with many steps. Fig. 18.1 illustrates the events that lead to an accident. Preliminary events can be anything which influences the initiating event. They set the stage for a hazardous condition. The initiating event is the actual mechanism or condition that causes the accident to occur. Intermediate events can have two effects. They may propagate or ameliorate the accident. Many times all of these steps have to be completed before an accident can occur. This is also illustrated by the so-called “accident triangle” shown in Fig. 18.2. The principle has been to consider the worst-case scenario and relate it to other major injuries, loss-of-time injuries, minor injuries, and noninjury accidents. It should be clear that for each major accident an enormous amount of minor and noninjury accidents happen, which can eventually add up to create a major incident. If the engineer cannot prevent one or more of these accident steps from occurring, then he can either prevent the mishap or at least mitigate its effects.
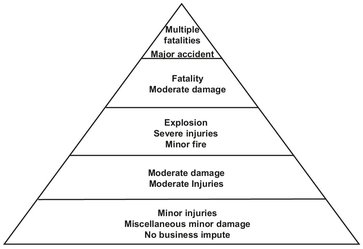
Fig. 18.2: The accident triangle.