Process Technology: An Introduction - Haan A.B. 2015
18 Process safety
18.3 Identification of hazardous properties of substances
18.3.1 Pure components
As illustrated by Fig. 18.4, the identification of the potential hazardous effects of a substance is the first step in the identification of hazards. The risk assessment of a chemical substance is a complex procedure based on comparison of its potential adverse effects with the reasonably foreseeable exposure of people and the environment to that substance. Hazard identification is mainly based on a critical evaluation of the intrinsic properties of a substance such as physicochemical, toxicological, and ecotoxicological properties. For each of these groups it is possible to define a number of specific end points, and for each of them internationally recognized guidelines to determine specific parameters. Interpretation of such parameters leads to the hazard identification of the chemicals. An important aid in the identification of potentially hazardous pure component properties is the so-called material safety data sheet (MSDS).
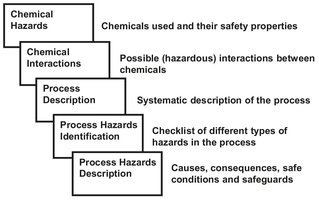
Fig. 18.4: Various steps in process hazard determination.
Physicochemical properties play an important role in the potential of a substance to produce adverse effects. Each physicochemical property plays a specific role. For instance, the molecular mass of the molecule gives an idea of whether the substance is bioavailable. A comparatively small molecule can enter an organism and produce its effect much more readily than a large molecule. The melting point characterizes the physical state of the substance. If the substance is a solid at room temperature, it is less mobile than a substance in a liquid or gaseous state. Like the melting point, the boiling point characterizes the physical state of the substance under normal pressure conditions. The lower the boiling point, the more easily the substance can be transferred into the air and the larger the risk of inhalatory effects. Relative density represents the mass ratio between a solid or liquid substance and water or a gaseous substance and air. When referred to water, its density indicates whether the substance can be expected to stratify on the surface or to sink to the bottom. Similarly for air, substances that are denser than air tend to stratify around the source of emission, while gases with a density lower than air diffuse easier into the air. Vapor pressure is also an important way to find out whether the substance may readily become volatile. A substance with a high vapor pressure chiefly affects inhalatory exposure, transfer potential, and risk of explosion. Water solubility has direct implications for the possibility of dissolution in surface waters, and therefore its mobility in water and soil.
Flammability provides direct information on the possibility of a so-called physical effect. The risk of flammability may derive from a low vapor ignition temperature in the presence of an ignition source or from the possibility of the substance igniting spontaneously in air with no ignition point. For liquid products, the flash point is the parameter that allows quantification of the flammability risk. It is defined as the lowest temperature at which a liquid emits vapors in such a quantity as to produce a flammable vapor/air mixture. Fig. 18.5 shows a setup for the experimental determination of flash points. The substance is slowly heated and its vapors are exposed to a spark at regular intervals. The lowest temperature at which the vapor/air mixture flashes is the flash point.
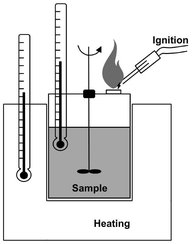
Fig. 18.5: Setup for flash point determination.
A gas explosion is a rapid chemical reaction (oxidation) in the gas phase which propagates through the explosive mixture in a self-sustaining process. The heat released leads rapidly to high temperatures, and can result in the buildup of high pressures. A gas explosion requires the presence of an explosive mixture consisting of a finely dispersed fuel in an oxidizer (oxygen, ozone, nitrogen dioxide) and the simultaneous presence of an effective ignition source. Any gaseous system containing mixed fuel and oxidizer is explosive only within a certain concentration range, the explosion range. In practice there is often only one fuel and air is the oxidizer. Here the explosion range can be characterized simply by stating the concentration limits on the fuel in air. These bounds, the lower explosion limit (LEL) and upper explosion limit (UEL), have been tabulated for a number of combustible gases and vapors. Nonexplosive mixtures in the range below the LEL are said to be too lean, and those above the UEL are said to be too rich.
A substance with oxidizing properties may bring about dangerous reactions causing fire, explosion, or the formation of other hazardous substances. Oxidizing properties are related to the potential of the substance to act as an oxidant in an oxidation-reduction reaction such as organic peroxides.
Toxicological properties are of direct interest in assessing the risk of chemical substances. They include a range of effects understood either as general poisoning of the organism or aimed at one or more particular target organs. The dosage administered and the duration of toxicological tests are inversely proportional with reference to acute, subacute, subchronic, and chronic studies. Acute toxicity includes those effects which may cause direct harm to health following a single exposure. It is common to distinguish between irreversible effects such as death and reversible effects such as irritation of the eyes and skin, or corrosive and sensitizing properties. With regard to acute toxic effects, substances are classified as very toxic, toxic, or harmful. This description is based on the median lethal dose LD50, at which half of the experimental animals die. Information on the more hidden effects that the substances may cause by exposure to low dosages over a period of time are provided by medium— or long-term toxicity tests. A basic aim of these tests is to identify the maximum dose level at which the substance does not present toxic effects. A substance is considered mutagenic if its adsorption may produce heritable genetic damage or increase their frequency. Similarly, substances are considered carcinogenic if they may produce cancer or increase its frequency. Teratogenic substances cause nonheritable birth defects. Ecotoxicological properties are indicators of the potential effects that a substance may have on the environment.
18.3.2 Exothermic chemical reactions
The majority of chemical reactions are accompanied by the production of heat (exothermic), which heats up the material itself, the reaction vessel, and the surroundings. The degree of heat dissipation depends on the heat capacity of the system itself and the heat removal capacity provided by the reaction vessel design and/or the latent heat of phase transitions. If the heat production rate exceeds the heat removal capacity of the system, the resulting temperature raise of the system will lead to a runaway reaction due to self-acceleration. The reaction velocity increases, more heat is produced per unit time, and the system continues to heat up more and more rapidly, until the maximum possible reaction velocity has been reached. The consequences of a runaway event can be manifold. One possibility is that the accompanying temperature rise may lead to solvent evaporation, with the subsequent possibility of a vapor cloud explosion or a dangerous pressure buildup by raising the vapor pressure of reactants. Another possibility is the formation of gaseous products from a decomposition reaction, which leads to a pressure increase and the risk of vessel rupture.
Tab. 18.1: Typical heats of reaction for common chemical reactions.
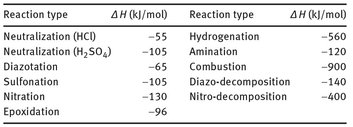
The overall heat of reaction and the rate of heat production determine the hazardous character of a chemical reaction. Some typical values for heats of reaction are summarized in Tab. 18.1. The chemical heat release of a reaction is controlled by the ability of the system to dissipate this energy. In mathematical terms this is formulated as a heat balance, including an accumulation term as well as heat generation and dissipation terms. A more simplified approach to illustrate the phenomena of runaway reaction systems is the Semenov model. Based on a pseudo-zero-order kinetic approach, the heat production rate QR is governed by an exponential dependence on temperature:
![]()
(18.1)
The rate of heat removal QC depends linearly on the driving temperature difference between the uniform reaction mass temperature and the ambient (jacket) temperature:
![]()
(18.2)
The controllability of the heat production rate can be explained by plotting the two heat flows as a function of temperature. As illustrated in Fig. 18.6, three different cases can be distinguished. In Case 1, small deviations from the steady state, represented by the lower of the two intersections, automatically result in a return to the origin. This is rated a stable operating point. In the upper steady state the original operation conditions are never reached again once a temperature deviation occurs. In the case of a temperature decrease, the process quickly approaches the lower steady state. For a temperature increase, the heat production rate always exceeds the heat removal capacity of the system. This leads to an unhindered self-acceleration of the reaction rate and thereby of the heat production rate. The same is true for all operating conditions of Case 3. Case 2 represents the limiting case of the first occurrence of an unstable operating point.
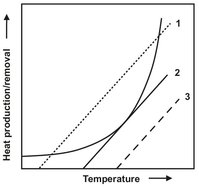
Fig. 18.6: Semenov plot.
In the runaway scenario it is assumed that a reactor is operated under normal operating conditions and a cooling failure occurs. In the case of a cooling failure, or if heat removal is not sufficient to compensate for the heat production, the temperature increases proportionally to the heat of reaction. Thus the reaction energy is a direct measure of the severity of a runaway. It is usually expressed as the adiabatic temperature increase Δ Tad the reaction mixture can exhibit when the full heat of reaction has to be accommodated by the reaction mixture temperature increase:
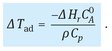
(18.3)
The adiabatic temperature increase is a valuable tool to estimate the dynamics of a runaway. As a general rule, high energies (ΔTad > 100 K) result in fast runaway or thermal explosion, while lower energies result in slower temperature increase rates. In practice three levels are used to classify the runaway hazard of a chemical reaction:
low: |
ΔTad < 50 K |
no pressure buildup; |
medium: |
50 K < ΔTad < 200 K; |
|
high: |
ΔTad > 200 K. |