Process Technology: An Introduction - Haan A.B. 2015
18 Process safety
18.4 Inherently safer plant design
18.4.1 The concept and its benefits
The term inherently safer implies that the process is safe because of its very nature and not because equipment has been added to make it more safe. Traditional plant designs try to reduce the risk by adding protective equipment and following safe methods of working. Nevertheless, this should be our second-best choice. Before we install safety equipment to control the consequences of a hazard, we should ask if the hazard can be eliminated. Plants should therefore be designed so that they are user-friendly and can tolerate departures from ideal performance and equipment failure without serious effects on safety, output, or efficiency.
The essence of the inherently safer approach to plant design is the avoidance of hazards rather than their control by adding protective equipment. To achieve this goal the planner should replace hazardous substances by less hazardous ones wherever possible. Large inventories should be avoided as far as possible. By applying the concept of inherently safer design at the very beginning of a project, we may be able to choose a safe product instead of a hazardous one. When a process is being chosen, we may be able to choose a route that avoids the use of hazardous raw materials or intermediates. Once the chemistry has been decided and we are developing a flow sheet, we may be able to choose or develop intensified equipment such as reactors and heat exchangers which do not require large quantities of materials in progress. When we get to the detailed design, we may be able to reduce inventories by the application of well-known methods.
Inherently safer plants are usually cheaper than conventional ones because they do not need as much additional protective equipment. Although it is difficult to say how much cheaper, on most plants we can expect to save at least 5 % of the capital cost for a new plant if we can reduce our inventories of hazardous materials. Equally important would be reductions in the cost of testing and maintaining the equipment. Finally the biggest savings from reducing inventories will probably come from a reduction in size of the plant items and a corresponding reduction in the size of the piping, structures, and foundations.
18.4.2 The road to friendlier plants
The first constraint on the road to friendlier plants is that company procedures do not usually ask for safety studies to be carried out early in designing. Safety advisors do not get involved and safety studies are not carried out until comparatively late in design. Then it is too late to make major changes and avoid hazards. All we can do is control them by adding protective equipment. However, when recognized in time, intensification, substitution, attenuation and simplification, schematically illustrated in Fig. 18.7, are valuable techniques which can result in an inherently safer design because hazards are avoided instead of controlled.
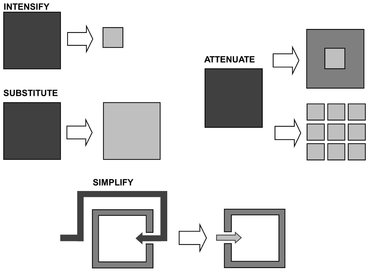
Fig. 18.7: The various roads to inherently safer plants. Adapted from [202].
Intensification or minimization
The best way of preventing large leaks of hazardous materials is not to have so much hazardous material about. Friendly plants contain low inventories of hazardous materials: so little that it does not matter if the entire inventory leaks out. What you don’t have can’t leak. This may seem obvious, but until recently little thought has been given to ways of reducing the amount of hazardous material in a plant. Engineers simply designed a plant and accepted whatever inventory the design required, confident that they could keep it under control. No unit operation offers more scope for reduction of inventory than reaction. Many continuous reactors, such as liquid-phase oxidation reactors, contain large inventories of highly flammable liquids. As a rule, reactors of all types are not large because a large output is desired, but because conversion is low, reaction is slow, or both. When conversion is low, most of the throughput has to be recovered and recycled, which increases the plant inventory. For example, nitroglycerin used to be made in batch reactors containing about 1 ton. Now it is made in small, continuous reactors containing a few hundred grams.
Substitution
If intensification is not possible, then an alternative is substitution: using a safer material in place of a hazardous one. Thus it may be possible to replace flammable refrigerants and heat transfer media by nonflammable ones, hazardous products by safer ones, and processes that use hazardous raw materials or intermediates by processes that do not. For instance water under pressure can be used as a heat transfer medium to replace flammable oils.
Attenuation
Another alternative to intensification is attenuation by using a hazardous material under less hazardous conditions. For example gaseous propylene is used to produce polypropylene instead of liquid propylene dissolved in a flammable solvent. Attenuation is sometimes the reverse of intensification, because if we make reaction conditions less extreme we may need a longer residence time and a larger inventory.
Simplify
Modern plants are very complicated, and this makes them expensive and provides too many opportunities for error. Simpler plants are friendlier than complex plants, because they provide fewer opportunities for error and less equipment that can fail and/or leak. Usually they are also cheaper. The main reason for complexity in plant design is the need to add equipment to control hazards. Inherently safer plants are therefore also simpler plants.
Nomenclature
A |
heat transfer area |
[m2] |
C |
concentration |
[mol m—3] |
EA |
activation energy |
[J mol—1 K—1] |
ΔHR |
heat of reaction |
[kJ mol—1] |
K∞ |
pre-exponential factor |
[s—1] |
Qc |
heat removal rate |
[kJ s—1] |
QR |
heat production rate |
[kJ s—1] |
R |
gas constant |
[8.314 J mol—1 K—1] |
T |
temperature |
[K] |
U |
heat transfer coefficient |
[W m—2 K—1] |
V |
volume |
[m3] |