CHEMISTRY THE CENTRAL SCIENCE
11 LIQUIDS AND INTERMOLECULAR FORCES
11.3 SELECT PROPERTIES OF LIQUIDS
The intermolecular attractions we have just discussed can help us understand many familiar properties of liquids. In this section we examine two: viscosity and surface tension.
Viscosity
Some liquids, such as molasses and motor oil, flow very slowly; others, such as water and gasoline, flow easily. The resistance of a liquid to flow is called viscosity. The greater a liquid's viscosity, the more slowly it flows. Viscosity can be measured by timing how long it takes a certain amount of the liquid to flow through a thin vertical tube (![]() FIGURE 11.17). Viscosity can also be determined by measuring the rate at which steel balls fall through the liquid. The balls fall more slowly as the viscosity increases.
FIGURE 11.17). Viscosity can also be determined by measuring the rate at which steel balls fall through the liquid. The balls fall more slowly as the viscosity increases.
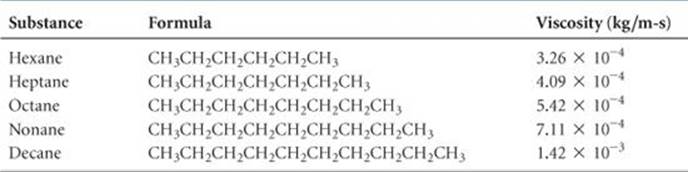
Viscosity is related to the ease with which the molecules of the liquid can move relative to one another. It depends on the attractive forces between molecules and on whether the shapes of the molecules are such that they tend to become entangled (for example, long molecules can become tangled like spaghetti). For a series of related compounds, viscosity increases with molecular weight, as illustrated in ![]() TABLE 11.4. The SI units for viscosity are kg/m-s. For any given substance, viscosity decreases with increasing temperature. Octane, for example, has a viscosity of 7.06 × 10–4 kg/m-s at 0 °C and 4.33 × 10–4 kg/m-s at 40 °C. At higher temperatures the greater average kinetic energy of the molecules overcomes the attractive forces between molecules.
TABLE 11.4. The SI units for viscosity are kg/m-s. For any given substance, viscosity decreases with increasing temperature. Octane, for example, has a viscosity of 7.06 × 10–4 kg/m-s at 0 °C and 4.33 × 10–4 kg/m-s at 40 °C. At higher temperatures the greater average kinetic energy of the molecules overcomes the attractive forces between molecules.

![]() FIGURE 11.17 Comparing viscosities. The Society of Automotive Engineers (SAE) has established a numeric scale to indicate motor-oil viscosity.
FIGURE 11.17 Comparing viscosities. The Society of Automotive Engineers (SAE) has established a numeric scale to indicate motor-oil viscosity.
Surface Tension
The surface of water behaves almost as if it had an elastic skin, as evidenced by the ability of certain insects to “walk” on water. This behavior is due to an imbalance of intermolecular forces at the surface of the liquid. As shown in ![]() FIGURE 11.18, molecules in the interior are attracted equally in all directions, but those at the surface experience a net inward force. This net force tends to pull surface molecules toward the interior, thereby reducing the surface area and making the molecules at the surface pack closely together.
FIGURE 11.18, molecules in the interior are attracted equally in all directions, but those at the surface experience a net inward force. This net force tends to pull surface molecules toward the interior, thereby reducing the surface area and making the molecules at the surface pack closely together.
Because spheres have the smallest surface area for their volume, water droplets assume an almost spherical shape. This explains the tendency of water to “bead up” when it contacts a surface made of nonpolar molecules, like a lotus leaf or a newly waxed car.
A measure of the net inward force that must be overcome to expand the surface area of a liquid is given by its surface tension. Surface tension is the energy required to increase the surface area of a liquid by a unit amount. For example, the surface tension of water at 20 °C is 7.29 × 10–2 J/m2, which means that an energy of 7.29 × 10–2 J must be supplied to increase the surface area of a given amount of water by 1 m2. Water has a high surface tension because of its strong hydrogen bonds. The surface tension of mercury is even higher (4.6 × 10–1 J/m2) because of even stronger metallic bonds between the atoms of mercury.
TABLE 11.4 • Viscosities of a Series of Hydrocarbons at 20 °C
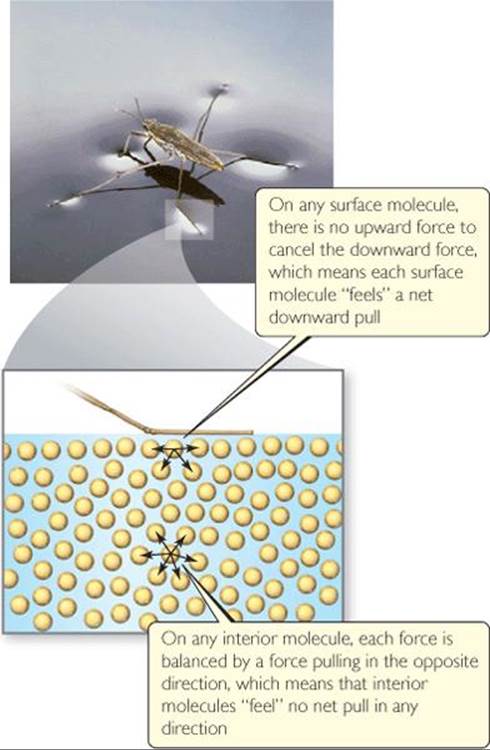
![]() FIGURE 11.18 Molecular-level view of surface tension. A water strider does not sink because of the high surface tension of water.
FIGURE 11.18 Molecular-level view of surface tension. A water strider does not sink because of the high surface tension of water.
![]() GO FIGURE
GO FIGURE
If the inside surface of each tube were coated with wax, would the general shape of the water meniscus change? Would the general shape of the mercury meniscus change?
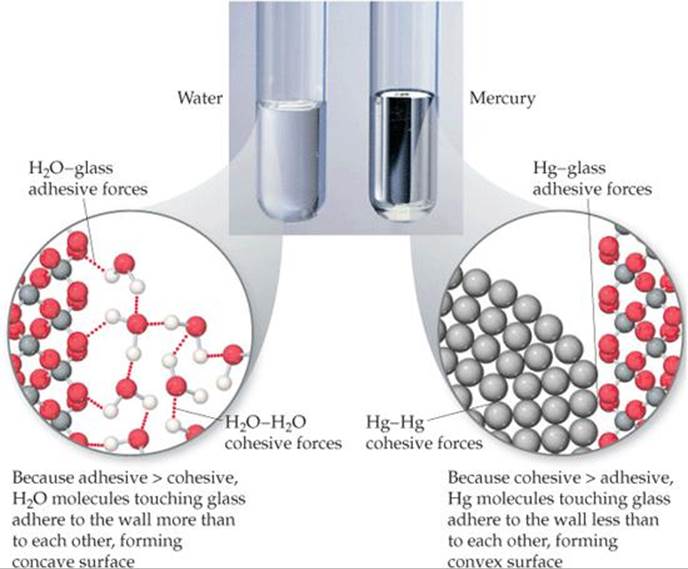
![]() FIGURE 11.19 Meniscus shapes for water and mercury in glass tubes.
FIGURE 11.19 Meniscus shapes for water and mercury in glass tubes.
![]() GIVE IT SOME THOUGHT
GIVE IT SOME THOUGHT
How do viscosity and surface tension change
a. as temperature increases,
b. as intermolecular forces of attraction become stronger?
Intermolecular forces that bind similar molecules to one another, such as the hydrogen bonding in water, are called cohesive forces. Intermolecular forces that bind a substance to a surface are called adhesive forces. Water placed in a glass tube adheres to the glass because the adhesive forces between the water and glass are greater than the cohesive forces between water molecules. The curved surface, or meniscus, of the water is therefore U-shaped (![]() FIGURE 11.19). For mercury, however, the situation is different. Mercury atoms can form bonds with one another but not with the glass. As a result the cohesive forces are much greater than the adhesive forces and the meniscus is shaped like an inverted U.
FIGURE 11.19). For mercury, however, the situation is different. Mercury atoms can form bonds with one another but not with the glass. As a result the cohesive forces are much greater than the adhesive forces and the meniscus is shaped like an inverted U.
When a small-diameter glass tube, or capillary, is placed in water, water rises in the tube. The rise of liquids up very narrow tubes is called capillary action. The adhesive forces between the liquid and the walls of the tube tend to increase the surface area of the liquid. The surface tension of the liquid tends to reduce the area, thereby pulling the liquid up the tube. The liquid climbs until the force of gravity on the liquid balances the adhesive and cohesive forces. Capillary action helps water and dissolved nutrients move upward through plants.