CHEMISTRY THE CENTRAL SCIENCE
2 ATOMS, MOLECULES, AND IONS
2.5 THE PERIODIC TABLE
As the list of known elements expanded during the early 1800s, attempts were made to find patterns in chemical behavior. These efforts culminated in the development of the periodic table in 1869. We will have much to say about the periodic table in later chapters, but it is so important and useful that you should become acquainted with it now. You will quickly learn that the periodic table is the most significant tool that chemists use for organizing and remembering chemical facts.
Many elements show strong similarities to one another. The elements lithium (Li), sodium (Na), and potassium (K) are all soft, very reactive metals, for example. The elements helium (He), neon (Ne), and argon (Ar) are all very nonreactive gases. If the elements are arranged in order of increasing atomic number, their chemical and physical properties show a repeating, or periodic, pattern. For example, each of the soft, reactive metals—lithium, sodium, and potassium—comes immediately after one of the non-reactive gases—helium, neon, and argon—as shown in ![]() FIGURE 2.14.
FIGURE 2.14.
![]() GO FIGURE
GO FIGURE
If F is a reactive nonmetal, which other element or elements shown here do you expect to also be a reactive nonmetal?
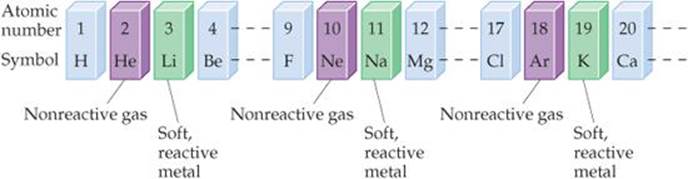
![]() Figure 2.14 Arranging elements by atomic number reveals a periodic pattern of properties. This pattern is the basis of the periodic table.
Figure 2.14 Arranging elements by atomic number reveals a periodic pattern of properties. This pattern is the basis of the periodic table.
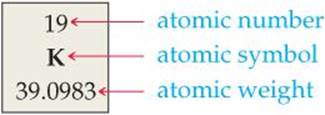
The arrangement of elements in order of increasing atomic number, with elements having similar properties placed in vertical columns, is known as the periodic table (![]() FIGURE 2.15). The table shows the atomic number and atomic symbol for each element, and the atomic weight is often given as well, as in this typical entry for potassium:
FIGURE 2.15). The table shows the atomic number and atomic symbol for each element, and the atomic weight is often given as well, as in this typical entry for potassium:
You may notice slight variations in periodic tables from one book to another or between those in the lecture hall and in the text. These are simply matters of style, or they might concern the particular information included. There are no fundamental differences.
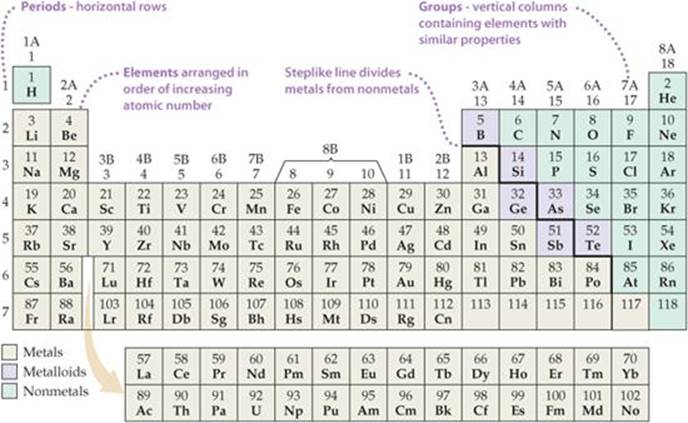
![]() Figure 2.15 Periodic table of the elements.
Figure 2.15 Periodic table of the elements.
The horizontal rows of the periodic table are called periods. The first period consists of only two elements, hydrogen (H) and helium (He). The second and third periods consist of eight elements each. The fourth and fifth periods contain 18 elements. The sixth period has 32 elements, but for it to fit on a page, 14 of these elements (atomic numbers 57-70) appear at the bottom of the table. The seventh period is incomplete, but it also has 14 of its members placed in a row at the bottom of the table.
The vertical columns are groups. The way in which the groups are labeled is somewhat arbitrary. Three labeling schemes are in common use, two of which are shown in Figure 2.15. The top set of labels, which have A and B designations, is widely used in North America. Roman numerals, rather than Arabic ones, are often employed in this scheme. Group 7A, for example, is often labeled VIIA. Europeans use a similar convention that numbers the columns from 1A through 8A and then from 1B through 8B, thereby giving the label 7B (or VIIB) instead of 7A to the group headed by fluorine (F). In an effort to eliminate this confusion, the International Union of Pure and Applied Chemistry (IUPAC) has proposed a convention that numbers the groups from 1 through 18 with no A or B designations, as shown in Figure 2.15. We will use the traditional North American convention with Arabic numerals and the letters A and B.
Elements in a group often exhibit similarities in physical and chemical properties. For example, the “coinage metals”—copper (Cu), silver (Ag), and gold (Au)—belong to group 1B. These elements are less reactive than most metals, which is why they are used throughout the world to make coins. Many other groups in the periodic table also have names, listed in ![]() TABLE 2.3.
TABLE 2.3.
We will learn in Chapters 6 and 7 that elements in a group have similar properties because they have the same arrangement of electrons at the periphery of their atoms. However, we need not wait until then to make good use of the periodic table; after all, chemists who knew nothing about electrons developed the table! We can use the table, as they intended, to correlate behaviors of elements and to help us remember many facts. The color code of Figure 2.15 shows that, except for hydrogen, all the elements on the left and in the middle of the table are metallic elements, or metals. All the metallic elements share characteristic properties, such as luster and high electrical and heat conductivity, and all of them except mercury (Hg) are solid at room temperature. The metals are separated from the nonmetallic elements, or nonmetals, by a stepped line that runs from boron (B) to astatine (At). (Note that hydrogen, although on the left side of the table, is a nonmetal.) At room temperature some of the nonmetals are gaseous, some are solid, and one is liquid. Nonmetals generally differ from the metals in appearance (![]() FIGURE 2.16) and in other physical properties. Many of the elements that lie along the line that separates metals from nonmetals have properties that fall between those of metals and those of nonmetals. These elements are often referred to as metalloids.
FIGURE 2.16) and in other physical properties. Many of the elements that lie along the line that separates metals from nonmetals have properties that fall between those of metals and those of nonmetals. These elements are often referred to as metalloids.

![]() Figure 2.16 Examples of metals (top) and nonmetals (bottom).
Figure 2.16 Examples of metals (top) and nonmetals (bottom).
![]() GIVE IT SOME THOUGHT
GIVE IT SOME THOUGHT
Chlorine is a halogen (Table 2.3). Locate this element in the periodic table.
a. What is its symbol?
b. In which period and in which group is the element located?
c. What is its atomic number?
d. Is it a metal or nonmetal?
TABLE 2.3 • Names of Some Groups in the Periodic Table

 A CLOSER LOOK
A CLOSER LOOK
GLENN SEABORG AND SEABORGIUM
Prior to 1940 the periodic table ended at uranium, element number 92. Since that time, no scientist has had a greater effect on the periodic table than Glenn Seaborg (![]() FIGURE 2.17). In 1940 Seaborg, Edwin McMillan, and coworkers at the University of California, Berkeley, succeeded in isolating plutonium (Pu) as a product of the reaction between uranium and neutrons. We will talk about reactions of this type, called nuclear reactions, in Chapter 21.
FIGURE 2.17). In 1940 Seaborg, Edwin McMillan, and coworkers at the University of California, Berkeley, succeeded in isolating plutonium (Pu) as a product of the reaction between uranium and neutrons. We will talk about reactions of this type, called nuclear reactions, in Chapter 21.
Between 1944 and 1958, Seaborg and his coworkers also identified various products of nuclear reactions as being the elements having atomic numbers 95 through 102. All these elements are radioactive and are not found in nature; they can be synthesized only via nuclear reactions. For their efforts in identifying the elements beyond uranium (the transuranium elements), McMillan and Seaborg shared the 1951 Nobel Prize in Chemistry.
From 1961 to 1971, Seaborg served as the chairman of the US Atomic Energy Commission (now the Department of Energy). In this position he had an important role in establishing international treaties to limit the testing of nuclear weapons. Upon his return to Berkeley, he was part of the team that in 1974 first identified element number 106. In 1994, to honor Seaborg's many contributions to the discovery of new elements, the American Chemical Society proposed that element number 106 be named seaborgium (Sg). After several years of controversy about whether an element should be named after a living person, the IUPAC officially adopted the name in 1997. Seaborg became the first person to have an element named after him while he was alive.

![]() Figure 2.17 Glenn Seaborg (1912–1999). Seaborg at Berkeley in 1941 measuring radiation produced by plutonium.
Figure 2.17 Glenn Seaborg (1912–1999). Seaborg at Berkeley in 1941 measuring radiation produced by plutonium.
RELATED EXERCISE: 2.95
SAMPLE EXERCISE 2.5 Using the Periodic Table
Which two of these elements would you expect to show the greatest similarity in chemical and physical properties: B, Ca, F, He, Mg, P?
SOLUTION
Elements in the same group of the periodic table are most likely to exhibit similar properties. We therefore expect Ca and Mg to be most alike because they are in the same group (2A, the alkaline earth metals).
PRACTICE EXERCISE
Locate Na (sodium) and Br (bromine) in the periodic table. Give the atomic number of each and classify each as metal, metalloid, or nonmetal.
Answer: Na, atomic number 11, is a metal; Br, atomic number 35, is a nonmetal.