Microreactors in Organic Chemistry and Catalysis, Second Edition (2013)
5. Homogeneous Reactions
5.8. Reaction with Organometallic Reagents
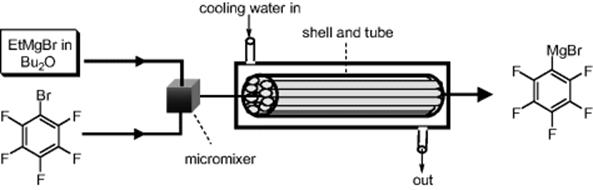
A Grignard exchange reaction was used for preparing Grignard reagents, which are difficult to prepare by reacting organic halides with metallic magnesium. Yoshida and coworkers aimed to perform the rapid and highly exothermic Grignard exchange reaction of ethylmagnesium bromide and bromopentafluorobenzene in a microflow system (Scheme 5.51) [69]. Different types of micromixers (T-shaped mixer, IMM multi-lamination mixer, and Toray Hi-mixer) were examined. A “shell and tube” micro-heat-exchanger, consisting of 55 microtubes (internal diameter = 490 μm, length = 200 mm) bundled together and placed in a shell (internal diameter = 16.7 mm, length = 200 mm), was employed for the residence time unit. By combining a Toray Hi-mixer with the shell and tube heat exchanger, multi-kilograms of the product were obtained after 24 h of continuous operation.
Scheme 5.51
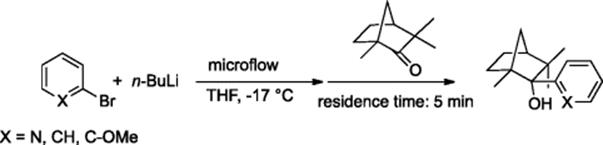
Halogen–lithium exchange reactions are common procedures in organic synthesis. However, large-scale use demands safety measures and strict temperature control. Since efficient control over exothermic reactions was accomplished by means of microreaction technology, Schmalz et al. investigated the microflow generation of aryllithium compounds from bromo-aromatics using n-BuLi and subsequent quenching with fenchone in THF at −17 °C (Scheme 5.52) [70]. This microflow protocol had a high yield (for example, when X = N, yield was 93%) and required a brief reaction time, for example, 5 min.
Scheme 5.52
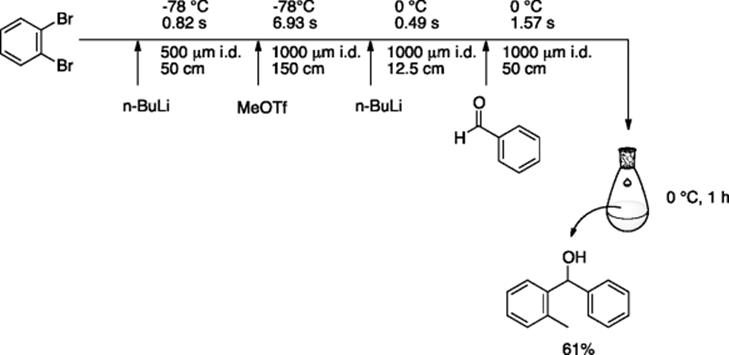
Chemistry of monolithiation of an aromatic ring possessing two halogen atoms, such as o-dibromobenzene, is quite challenging, because the intermediate o-bromophenyllithium is highly unstable and easily decomposes to benzyne, even at −78 °C. Yoshida and coworkers employed a very simple tube and T-mixer-based microreaction system where o-bromophenyllithium is generated and immediately reacted with an electrophile within a sub-second (0.8 s) residence time, thereby escaping any decomposition [71]. Combination of four micromixers and four tube reactors in a series provided the means for double electrophilic substitution of o-dibromobenzene via sequential lithiation (Scheme 5.53).
Scheme 5.53
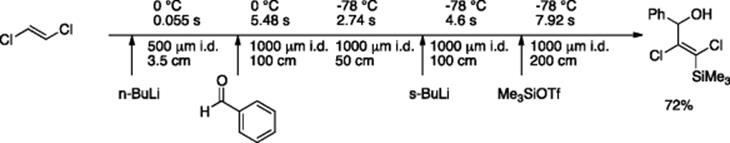
This concept of flash chemistry was extended for the generation of 2-chlorovinyllithium from 1,2-dichloroethane and butyllithium [72]. Typically, such reactions are carried out at −78 °C to avoid decomposition of intermediates via β-elimination; however, it was possible to carry out the microfluidic generation of 2-chlorovinyllithium and subsequent reactions with numerous electrophiles even at 0 °C by shortening the residence time up to 0.055 s (Scheme 5.54). By intentionally prolonging the residence time over 50 s, and thereby allowing the β-elimination to take place, the authors were able to generate both symmetrically and unsymmetrically disubstituted alkyne.
Scheme 5.54
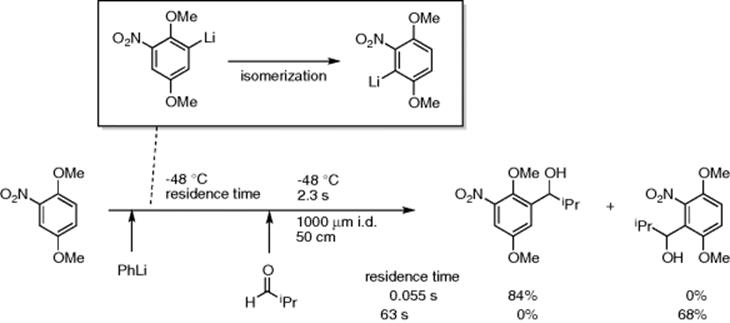
Another related and interesting demonstration from this group was the residence time-guided switch between kinetic and thermodynamic products when nitro-substituted arylhalides were reacted with phenyllithium. They showed that the initially formed aryllithium gave the kinetic product if quenched with an aldehyde within a 0.06 s residence time, while an extended residence time of 63 s allowed the intermediate to isomerize and produced, exclusively, the thermodynamic product with an aldehyde (Scheme 5.55) [73]. In a subsequent paper, they also showed that microfluidic asymmetric synthesis involving an unstable chiral organolithium intermediate can benefit from the fine-tuning of residence time that helped suppressing undesirable epimerization of the intermediate [74].
Scheme 5.55
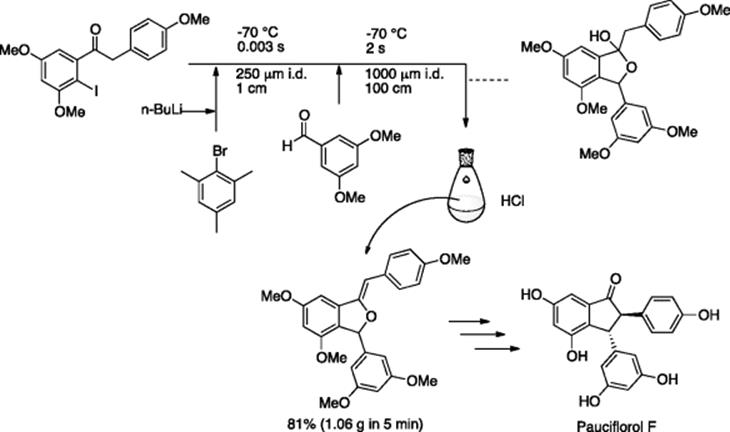
Similar to the nitro-substituent, an aryl halide that contains a carbonyl group (C=O) poses a formidable challenge in lithiation-electrophilic addition chemistry, because the carbonyl group itself is an electrophile and thus requires a protecting group. Yoshida et al. presented an integrated microflow system that, with a millisecond (0.003 s or less) reaction time window, enabled a protection group-free generation and trapping of short-lived acylphenyllithium species This method was adapted as a key step in the synthesis of naturally occurring compound, Pauciflorol (Scheme 5.56) [75].
Scheme 5.56
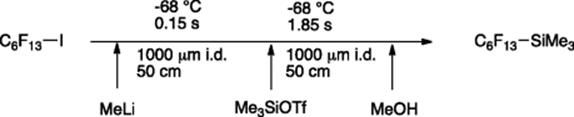
The Yoshida's group further explored the scope of microflow-lithiation chemistry for perfluorinated alkyl halides with methyllithium [76]. In this work, rapid micromixing enabled the in situ generation and consumption of perfluoroalkyllithium in the presence of an electrophile within 3.74 s of residence time and at 0 °C. For the analogous batch reaction, lowering the temperature to −78 °C would be required to avoid the decomposition of the intermediate via β-elimination. Use of electrophiles that are highly sensitive to methyllithium (e.g., chlorotributylstannate, trimethylsilyltriflate, and isocyanates) was also possible by performing the three key steps: lithiation, trapping (with electrophile), and quenching in three reactor segments of an integrated microflow network (Scheme 5.57).
Scheme 5.57
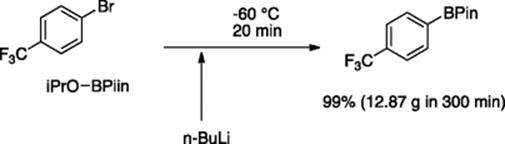
The Ley group employed a cryostatic flow reactor that is capable of maintaining the temperature at -80 °C for a prolonged period of time. The reactor design consists of two pre-cooling loops for two reactants, a T-mixer for mixing and a long tube-reactor part, wound around a cooled metal casing [77]. They tested the device for the lithiation-based synthesis of aryl boronates that are useful precursors in the transition metal-catalyzed CߝC, CߝN, and CߝO bond forming reactions. By employing piston-based pumps (K-120 type), they managed to flow 1.6 M n-butyllithium (in hexane) directly from the reagent bottle to the cryo- flow reactor system (kept at −60 °C) where it reacted with p-F3C-Ph-Br to generate p-F3C-Ph-Li that immediately reacted with pinacol boronate to give the desired product. A continuous operation for 5 h provided 12.87 g of the products with 99% yield (Scheme 5.58).
Scheme 5.58
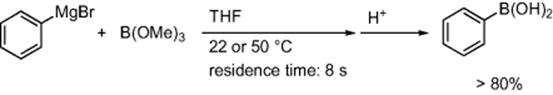
Typical industrial process for the synthesis of phenylboronic acid from phenylmagnesium bromide and boronic acid trimethoxy ester requires strict temperature control (−25 to −55 °C) to minimize the formation of side products. Recently, Hessel and coworkers reported that a micromixer (width; 40 μm, depth; 300 μm)/tubular reactor-system gave the phenylboronic acid in a high yield (>80%) even at higher temperatures (22 or 50 °C) with minimum amounts of the side products (Scheme 5.59) [78]. They also achieved a pilot-scale production by employing a caterpillar minimixer (width range, 600–1700 μm; width range, 1200–2400 μm).
Scheme 5.59
Microreaction technology has already shown a great deal of promises for homogeneous reactions, be thermal, photochemical, or electrochemical methods. Efficient mixing, precise control of reaction temperature, and residence time enable one to manipulate the selectivity issue, tame an ultrafast reaction, or even conduct a highly exothermic reaction at room temperature. To conclude, doors for many fascinating properties of microreaction technology are now open for organic chemists.
References
1. Panke, G., Schwalbe, T., Stirner, W., Taghavi-Moghadam, S., and Wille, G. (2003) Synthesis, 2827.
2. Ducry, L. and Roberge, D.M. (2005) Angew. Chem., Int. Ed., 44, 7972.
3. Tanaka, K., Motomatsu, S., Koyama, K., Tanaka, S.i., and Fukase, K. (2007) Org. Lett., 9, 299.
4. Ratner, D.M., Murphy, E.R., Jhunjhunwala, M., Snyder, D.A., Jensen, K.F., and Seeberger, P.H. (2005) Chem. Commun., 578.
5. Tanaka, K., Miyagawa, T., and Fukase, K. (2009) Synlett, 1571.
6. Sniady, A., Bedore, M.W., and Jamison, T.F. (2011) Angew. Chem., Int. Ed., 50, 2155.
7. Nishiyama, H., Kondo, M., Nakamura, T., and Itoh, K. (1991) Organometallics, 10, 500.
8. Jönsson, C., Lundgren, S., Haswell, S.J., and Moberg, C. (2004) Tetrahedron, 60, 10515.
9. Mikami, K., Yamanaka, M., Islam, M.N., Tonoi, T., Itoh, Y., Shinoda, M., and Kudo, K. (2006) J. Fluorine Chem., 127, 592.
10. Ikushima, Y., Hatakeda, K., Sato, M., Sato, O., and Arai, M. (2002) Chem. Commun., 2208.
11. Min, K.-I., Lee, T.-H., Park, C.P., Wu, Z.–Y., Girault, H.H., Ryu, I., Fukuyama, T., Mukai, Y., and Kim, D.-P. (2010) Angew. Chem., Int. Ed., 49, 7063.
12. Bogdan, A.R., Poe, S.L., Kubis, D.C., Broadwater, S.J., and McQuade, D.T. (2009) Angew. Chem., Int. Ed., 48, 8547.
13. Kuwajima, I. and Nakamura, E. (1985) Acc. Chem. Res., 18, 181.
14. Wiles, C., Watts, P., Haswell, S.J., and Pombo-Villar, E. (2002) Chem. Commun., 1034.
15. Wiles, C., Watts, P., Haswell, S.J., and Pombo-Villar, E. (2005) Tetrahedron, 61, 10757.
16. Löwe, H., Hessel, V., Löb, P., and Hubbard, S. (2006) Org. Process Res. Dev., 10, 1144.
17. Wiles, C., Watts, P., Haswell, S.J., and Pombo-Villar, E. (2004) Lab Chip, 4, 171.
18. Acke, D.R.J. and Stevens, C.V. (2006) Org. Process Res. Dev., 10, 417.
19. Miyake, N. and Kitazume, T. (2003) J. Fluorine Chem., 122, 243.
20. Kawai, K., Ebata, T., and Kitazume, T. (2005) J. Fluorine Chem., 126, 956.
21. Fukuyama, T., Kobayashi, M., Rahman, M.T., Kamata, N., and Ryu, I. (2008) Org. Lett., 10, 533.
22. Odedra, A., Geyer, K., Gustafsson, T., Gilmour, R., and Seeberger, P.H. (2008) Chem. Commun., 3025.
23. Wienhöfer, I.C., Studer, A., Rahman, M.T., Fukuyama, T., and Ryu, I. (2009) Org. Lett., 11, 2457.
24. Skelton, V., Greenway, G.M., Haswell, S.J., Styring, P., Morgan, D.O., Warrington, B.H., and Wong, S.Y.F. (2001) Analyst, 126, 7.
25. Skelton, V., Greenway, G.M., Haswell, S.J., Styring, P., Morgan, D.O., Warrington, B.H., and Wong, S.Y.F. (2001) Analyst, 126, 11.
26. Sands, M., Haswell, S.J., Kelly, S.M., Skelton, V., Morgan, D.O., Styring, P., and Warrington, B. (2001) Lab Chip, 1, 64.
27. Wiles, C., Watts, P., Haswell, S.J., and Pombo-Villar, E. (2003) Tetrahedron, 59, 10173.
28. Webb, D. and Jamison, T.F. (2012) Org. Lett, 14, 568.
29. Webb, D. and Jamison, T.F. (2012) Org. Lett., 14, 2465.
30. Garcia-Egido, E., Wong, S.Y.F., and Warrington, B.H. (2002) Lab Chip, 2, 31.
31. Wiles, C., Watts, P., and Haswell, S.J. (2004) Org. Process Res. Dev., 8, 28.
32. Schwalbe, T., Autze, V., Hohmann, M., and Stirner, W. (2004) Org. Process Res. Dev., 8, 440.
33. Fernandez-Suarez, M., Wong, S.Y.F., and Warrington, B.H. (2002) Lab Chip, 2, 170.
34. Grant, D., Dahl, R., and Cosford, N.D.P. (2008) J. Org. Chem., 73, 7219.
35. Herath, A. and Cosford, N.D.P. (2010) Org. Lett., 12, 5182.
36. Herath, A., Dahl, R., and Cosford, N.D.P. (2010) Org. Lett., 12, 412.
37. Bremner, W.S. and Organ, M.G. (2007) J. Comb. Chem., 9, 14.
38. Obermeyer, D., Glasnov, T.N., and Kappe, C.O. (2011) J. Org. Chem., 76, 6657.
39. Gustafsson, T., Pontén, F., and Seeberger, P.H. (2008) Chem. Commun., 1100.
40. Fuse, S., Tanabe, N., and Takahashi, T. (2011) Chem. Commun., 12661.
41. Watts, P., Wiles, C., Haswell, S.J., Pombo-Viller, E., and Styring, P. (2001) Chem. Commun., 990.
42. Watts, P., Wiles, C., Haswell, S.J., and Pombo-Viller, E. (2001) Tetrahedron, 58, 5427.
43. Watts, P., Wiles, C., Haswell, S.J., and Pombo-Viller, E. (2002) Lab Chip, 2, 141.
44. Flögel, O., Codée, J.D.C., Seebach, D., and Seeberger, P.H. (2006) Angew. Chem., Int. Ed., 45, 7000.
45. Fukuyama, T., Shinmen, M., Nishitani, S., Sato, M., and Ryu, I. (2002) Org. Lett., 4, 1691.
46. Rahman, M.T., Fukuyama, T., Kamata, N., Sato, M., and Ryu, I. (2006) Chem. Commun., 2236.
47. Liu, S., Fukuyama, T., Sato, M., and Ryu, I. (2004) Org. Process Res. Dev., 8, 477.
48. Sugimoto, A., Fukuyama, T., Rahman, M.T., and Ryu, I. (2009) Tetrahedron Lett., 50, 6364.
49. Fukuyama, T., Rahman, M.T., Sumino, Y., and Ryu, I. (2012) Synlett, 2279.
50. Zhang, Y., Jamison, T.F., Patel, S., and Mainolfi, N. (2011) Org. Lett., 13, 280.
51. Basheer, C., Hussain, F.S.J., Lee, H.K., and Valiyaveettil, S. (2004) Tetrahedron Lett., 45, 7297.
52. Comer, E. and Organ, M.G. (2005) J. Am. Chem. Soc., 127, 8160.
53. Shu, W., Pellegatti, L., Oberli, M.A., and Buchwald, S.L. (2011) Angew. Chem., Int. Ed., 50, 10665.
54. Shu, W. and Buchwald, S.L. (2012) Angew. Chem., Int. Ed., 51, 5355.
55. Nagaki, A., Kenmoku, A., Moriwaki, Y., Hayashi, A., and Yohida, J. (2010) Angew. Chem., Int. Ed., 49, 7543.
56. Mauger, C., Buisine, O., Caravieilhes, S., and Mignani, G. (2005) J. Organomet. Chem., 690, 3627.
57. Basheer, C., Vetrichelvan, M., Suresh, V., and Lee, H.K. (2006) Tetrahedron Lett., 47, 957.
58. Monfette, S., Eyholzer, M., Roberge, D.M., and Fogg, D.E. (2010) Chem. Eur. J., 16, 11720.
59. Park, C.P., Van Wingerden, M.M., Han, S.-Y., Kim, D.-P., and Grubbs, R.H. (2011) Org. Lett., 13, 2398.
60. Razzaq, T., Glasnov, T.N., and Kappe, C.O. (2009) Eur. J. Org. Chem., 1321.
61. Kong, L., Lin, Q., Lv, X., Yang, Y., Jia, Y., and Zhou, Y. (2009) Green Chem., 11, 1108.
62. Gutmann, B., Roduit, J.-P., Roberge, D., and Kappe, C.O. (2010) Angew. Chem., Int. Ed., 49, 7101.
63. Palde, P.B. and Jamison, T.F. (2011) Angew. Chem., Int. Ed., 50, 3525.
64. O'Brein, A.G., Lévesque, F., and Seeberger, P.H. (2011) Chem. Commun., 47, 2688.
65. Shore, G. and Organ, M.G. (2008) Chem. Commun., 838.
66. Ceylan, S., Coutable, L., Wegner, J., and Kirschning, A. (2011) Chem. Eur. J., 17, 1884.
67. Kawaguchi, T., Miyata, H., Ataka, K., Mae, K., and Yoshida, J. (2005) Angew. Chem., Int. Ed., 44, 2413.
68. Sedelmeier, J., Ley, S.V., Baxendale, I.R., and Baumann, M. (2010) Org. Lett., 12, 3618.
69. Wakami, H. and Yoshida, J. (2005) Org. Process Res. Dev., 9, 787.
70. El Sheikh, S. and Schmalz, H.G. (2004) Curr. Opin. Drug Discov. Dev., 7, 882.
71. Usutani, H., Tomida, Y., Nagaki, A., Okamoto, H., Nokami, T., and Yoshida, J. (2007) J. Am. Chem. Soc., 129, 3046.
72. Nagaki, A., Matsuo, C., Kim, S., Saito, K., Miyazaki, A., and Yoshida, J. (2012) Angew. Chem., Int. Ed., 51, 3245.
73. Nagaki, A., Kim, H., and Yoshida, J. (2009) Angew. Chem., Int. Ed., 48, 8063.
74. Tomida, Y., Nagaki, A., and Yoshida, J. (2011) J. Am. Chem. Soc., 133, 3744.
75. Kim, H., Nagaki, A., and Yoshida, J. (2011) Nat. Commun., 2, Article No. 264. doi: 10.1038/ncomms1264
76. Nagaki, A., Tokuoka, S., Yamada, S., Tomida, Y., Oshiro, K., Amii, H., and Yoshida, J. (2011) Org. Biomol. Chem., 9, 7559.
77. Browne, D.L., Baumann, M., Harji, B.H., Baxendale, I.R., and Ley, S.L. (2011) Org. Lett., 13, 3312.
78. Hessel, V., Hofmann, C., Löwe, H., Meudt, A., Scheres, S., Schönfeld, F., and Werner, B. (2004) Org. Process Res. Dev., 8, 511.