CHEMISTRY THE CENTRAL SCIENCE
18 CHEMISTRY OF THE ENVIRONMENT

THE BIG ISLAND of Hawaii.
WHAT'S AHEAD
18.1 EARTH'S ATMOSPHERE
We begin with a look at the temperature profile, pressure profile, and chemical composition of Earth's atmosphere. We then examine photoionization and photodissociation, reactions that result from atmospheric absorption of solar radiation.
18.2 HUMAN ACTIVITIES AND EARTH'S ATMOSPHERE
We next examine the effect human activities have on the atmosphere. We discuss how atmospheric ozone is depleted by reactions involving human-made gases and how acid rain and smog are the result of atmospheric reactions involving compounds produced by human activity.
18.3 EARTH'S WATER
We examine the global water cycle, which describes how water moves from the ground to surface water to the atmosphere and back into the ground. We compare the chemical compositions of seawater, freshwater, and groundwater.
18.4 HUMAN ACTIVITIES AND EARTH'S WATER
We consider how Earth's water is connected to the global climate and examine one measure of water quality: dissolved oxygen concentration. Water for drinking and for irrigation must be free of salts and pollutants.
18.5 GREEN CHEMISTRY
We conclude by examining green chemistry, an international initiative to make industrial products, processes, and chemical reactions compatible with a sustainable society and environment.
THE RICHNESS OF LIFE ON Earth, represented in the chapter-opening photograph, is, as far as we know, unique. Earth's atmosphere, the energy received from the Sun, and the abundance of water on our planet are all features currently believed to be necessary for life.
As technology has advanced and the world human population has increased, humans have put new and greater stresses on the environment. Paradoxically, the very technology that can cause pollution also provides the tools to help understand and manage the environment in a beneficial way. Chemistry is often at the heart of environmental issues. The economic growth of both developed and developing nations depends critically on chemical processes that range from treatment of water supplies to industrial processes. Some of these processes produce products or by-products that are harmful to the environment.
We are now in a position to apply the principles we have learned in preceding chapters to an understanding of how our environment operates and how human activities affect it. To understand and protect the environment in which we live, we must understand how human-made and natural chemical compounds interact on land and in the sea and sky. Our daily decisions as consumers mirror those of leading experts and governmental leaders: In making each decision, we must weigh the costs versus the benefits of our actions. Unfortunately, the environmental impacts of our decisions are often subtle and not immediately evident.
18.1 EARTH'S ATMOSPHERE
Because most of us have never been very far from Earth's surface, we often take for granted the many ways in which the atmosphere determines the environment in which we live. In this section we examine some of the important characteristics of our planet's atmosphere.
The temperature of the atmosphere varies with altitude (![]() FIGURE 18.1), and the atmosphere is divided into four regions based on this temperature profile. Just above the surface, in the troposphere, the temperature normally decreases with increasing altitude, reaching a minimum of about 215 K at about 10 km. Nearly all of us live our entire lives in the troposphere. Howling winds and soft breezes, rain, and sunny skies—all that we normally think of as “weather"—occur in this region. Commercial jet aircraft typically fly about 10 km (33,000 ft) above Earth, an altitude that defines the upper limit of the troposphere, which we call the tropopause.
FIGURE 18.1), and the atmosphere is divided into four regions based on this temperature profile. Just above the surface, in the troposphere, the temperature normally decreases with increasing altitude, reaching a minimum of about 215 K at about 10 km. Nearly all of us live our entire lives in the troposphere. Howling winds and soft breezes, rain, and sunny skies—all that we normally think of as “weather"—occur in this region. Commercial jet aircraft typically fly about 10 km (33,000 ft) above Earth, an altitude that defines the upper limit of the troposphere, which we call the tropopause.
Above the tropopause, air temperature increases with altitude, reaching a maximum of about 275 K at about 50 km. The region from 10 km to 50 km is the stratosphere, and above it are the mesosphere and thermosphere. Notice in Figure 18.1 that the temperature extremes that form the boundaries between adjacent regions are denoted by the suffix -pause. The boundaries are important because gases mix across them relatively slowly. For example, pollutant gases generated in the troposphere pass through the tropopause and find their way into the stratosphere only very slowly.
Atmospheric pressure decreases with increasing elevation (Figure 18.1), declining much more rapidly at lower elevations than at higher ones because of the atmosphere's compressibility. Thus, the pressure decreases from an average value of 760 torr (101 kPa) at sea level to 2.3 × 10–3torr (3.1 × 10–4 kPa) at 100 km, to only 1.0 × 10–6 torr (1.3 × 10–7 kPa) at 200 km.
![]() GO FIGURE
GO FIGURE
At what altitude is the atmospheric temperature lowest?
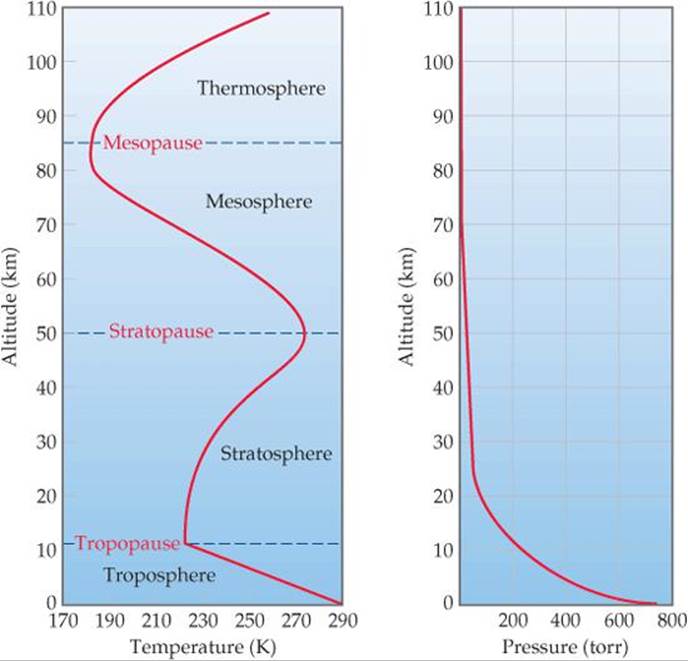
![]() FIGURE 18.1 Temperature and pressure in the atmosphere vary as a function of altitude above sea level.
FIGURE 18.1 Temperature and pressure in the atmosphere vary as a function of altitude above sea level.
The troposphere and stratosphere together account for 99.9% of the mass of the atmosphere, 75% of which is the mass in the troposphere. Consequently, most of the chemistry that follows focuses on these two regions.
Composition of the Atmosphere
Earth's atmosphere is constantly bombarded by radiation and energetic particles from the Sun. This barrage of energy has profound chemical and physical effects, especially in the upper regions of the atmosphere, above about 80 km (![]() FIGURE 18.2). In addition, because of Earth's gravitational field, heavier atoms and molecules tend to sink in the atmosphere, leaving lighter atoms and molecules at the top of the atmosphere. (This is why, as just noted, 75 percent of the atmosphere's mass is in the troposphere.) Because of all these factors, the composition of the atmosphere is not uniform.
FIGURE 18.2). In addition, because of Earth's gravitational field, heavier atoms and molecules tend to sink in the atmosphere, leaving lighter atoms and molecules at the top of the atmosphere. (This is why, as just noted, 75 percent of the atmosphere's mass is in the troposphere.) Because of all these factors, the composition of the atmosphere is not uniform.
![]() TABLE 18.1 shows the composition of dry air near sea level. Note that although traces of many substances are present, N2 and O2 make up about 99% of sea-level air. The noble gases and CO2 make up most of the remainder.
TABLE 18.1 shows the composition of dry air near sea level. Note that although traces of many substances are present, N2 and O2 make up about 99% of sea-level air. The noble gases and CO2 make up most of the remainder.
TABLE 18.1 • The Major Components of Dry Air Near Sea Level
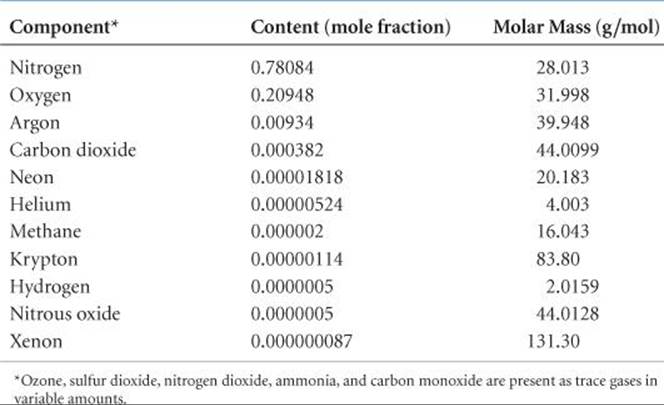
When applied to substances in aqueous solution, the concentration unit parts per million refers to grams of substance per million grams of solution. ![]() (Section 13.4) When dealing with gases, however, 1 ppm means one part by volume in 1 million volumes of the whole. Because volume is proportional to number of moles of gas via the ideal-gas equation (PV = nRT), volume fraction and mole fraction are the same. Thus, 1 ppm of a trace constituent of the atmosphere amounts to 1 mol of that constituent in 1 million moles of air; that is, the concentration in parts per million is equal to the mole fraction times 106. For example, Table 18.1 lists the mole fraction of CO2 in the atmosphere as 0.000382, which means its concentration in parts per million is 0.000382 × 106 = 382 ppm.
(Section 13.4) When dealing with gases, however, 1 ppm means one part by volume in 1 million volumes of the whole. Because volume is proportional to number of moles of gas via the ideal-gas equation (PV = nRT), volume fraction and mole fraction are the same. Thus, 1 ppm of a trace constituent of the atmosphere amounts to 1 mol of that constituent in 1 million moles of air; that is, the concentration in parts per million is equal to the mole fraction times 106. For example, Table 18.1 lists the mole fraction of CO2 in the atmosphere as 0.000382, which means its concentration in parts per million is 0.000382 × 106 = 382 ppm.
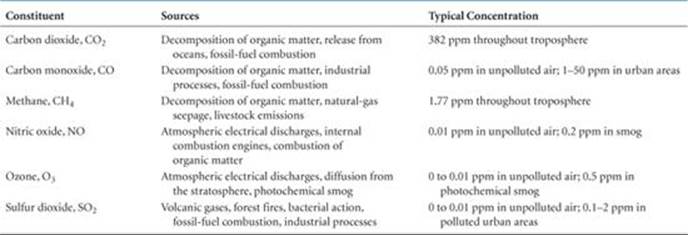
Other minor constituents of the troposphere, in addition to CO2, are listed in ![]() TABLE 18.2.
TABLE 18.2.
Before we consider the chemical processes that occur in the atmosphere, let's review some of the properties of the two major components, N2 and O2. Recall that the N2 molecule possesses a triple bond between the nitrogen atoms. ![]() (Section 8.3) This very strong bond (bond energy 941 kJ/mol) is largely responsible for the very low reactivity of N2. The bond energy in O2 is only 495 kJ/mol, making O2 much more reactive than N2. For example, oxygen reacts with many substances to form oxides. The oxides of nonmetals, such as SO2, usually form acidic solutions when dissolved in water. The oxides of active metals, such as CaO, form basic solutions when dissolved in water.
(Section 8.3) This very strong bond (bond energy 941 kJ/mol) is largely responsible for the very low reactivity of N2. The bond energy in O2 is only 495 kJ/mol, making O2 much more reactive than N2. For example, oxygen reacts with many substances to form oxides. The oxides of nonmetals, such as SO2, usually form acidic solutions when dissolved in water. The oxides of active metals, such as CaO, form basic solutions when dissolved in water. ![]() (Section 7.7)
(Section 7.7)

![]() FIGURE 18.2 The aurora borealis (northern lights).
FIGURE 18.2 The aurora borealis (northern lights).
TABLE 18.2 • Sources and Typical Concentrations of Some Minor Atmospheric Constituents
SAMPLE EXERCISE 18.1 Calculating the Concentration of Water in Air
What is the concentration, in parts per million, of water vapor in a sample of air if the partial pressure of the water is 0.80 torr and the total pressure of the air is 735 torr?
SOLUTION
Analyze We are given the partial pressure of water vapor and the total pressure of an air sample and asked to determine the water vapor concentration.
Plan Recall that the partial pressure of a component in a mixture of gases is given by the product of its mole fraction and the total pressure of the mixture ![]() (Section 10.6):
(Section 10.6):
![]()
Solve Solving for the mole fraction of water vapor in the mixture, XH2O, gives

The concentration in ppm is the mole fraction times 106:
![]()
PRACTICE EXERCISE
The concentration of CO in a sample of air is 4.3 ppm. What is the partial pressure of the CO if the total air pressure is 695 torr?
Answer: 3.0 × 10–3 torr
Photochemical Reactions in the Atmosphere
Although the atmosphere beyond the stratosphere contains only a small fraction of the atmospheric mass, it forms the outer defense against the hail of radiation and high-energy particles that continuously bombard Earth. As the bombarding radiation passes through the upper atmosphere, it causes two kinds of chemical changes: photodissociation and photoionization. These processes protect us from high-energy radiation by absorbing most of the radiation before it reaches the troposphere. If it were not for these photochemical processes, plant and animal life as we know it could not exist on Earth.
The Sun emits radiant energy over a wide range of wavelengths (![]() FIGURE 18.3). To understand the connection between the wavelength of radiation and its effect on atoms and molecules, recall that electromagnetic radiation can be pictured as a stream of photons.
FIGURE 18.3). To understand the connection between the wavelength of radiation and its effect on atoms and molecules, recall that electromagnetic radiation can be pictured as a stream of photons. ![]() (Section 6.2) The energy of each photon is given by E = hv, where h is Planck's constant and v is the radiation frequency. For a chemical change to occur when radiation strikes atoms or molecules, two conditions must be met. First, the incoming photons must have sufficient energy to break a chemical bond or remove an electron from the atom or molecule. Second, the atoms or molecules being bombarded must absorb these photons. When these requirements are met, the energy of the photons is used to do the work associated with some chemical change.
(Section 6.2) The energy of each photon is given by E = hv, where h is Planck's constant and v is the radiation frequency. For a chemical change to occur when radiation strikes atoms or molecules, two conditions must be met. First, the incoming photons must have sufficient energy to break a chemical bond or remove an electron from the atom or molecule. Second, the atoms or molecules being bombarded must absorb these photons. When these requirements are met, the energy of the photons is used to do the work associated with some chemical change.
![]() GO FIGURE
GO FIGURE
Why doesn't the solar spectrum at sea level perfectly match the solar spectrum outside the atmosphere?
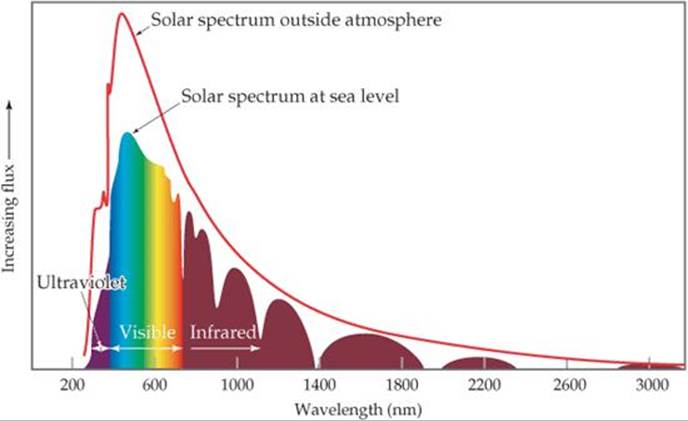
![]() FIGURE 18.3 The solar spectrum above Earth's atmosphere compared to that at sea level. The more structured curve at sea level is due to gases in the atmosphere absorbing specific wavelengths of light. “Flux,” the unit on the vertical axis, is light energy per area per unit of time.
FIGURE 18.3 The solar spectrum above Earth's atmosphere compared to that at sea level. The more structured curve at sea level is due to gases in the atmosphere absorbing specific wavelengths of light. “Flux,” the unit on the vertical axis, is light energy per area per unit of time.
The rupture of a chemical bond resulting from absorption of a photon by a molecule is called photodissociation. No ions are formed when the bond between two atoms is cleaved by photodissociation. Instead, half the bonding electrons stay with one atom and half stay with the other atom. The result is two electrically neutral particles.
One of the most important processes occurring above an altitude of about 120 km is photodissociation of the oxygen molecule:
![]()
The minimum energy required to cause this change is determined by the bond energy (or dissociation energy) of O2, 495 kJ/mol.
SAMPLE EXERCISE 18.2 Calculating the Wavelength Required to Break a Bond
What is the maximum wavelength of light, in nanometers, that has enough energy per photon to dissociate the O2 molecule?
SOLUTION
Analyze We are asked to determine the wavelength of a photon that has just enough energy to break the O═O double bond in O2.
Plan We first need to calculate the energy required to break the O═O double bond in one molecule and then find the wavelength of a photon of this energy.
Solve The dissociation energy of O2 is 495 kJ/mol. Using this value and Avogadro's number, we can calculate the amount of energy needed to break the bond in a single O2 molecule:

We next use the Planck relationship, E = hv, ![]() (Equation 6.2) to calculate the frequency v of a photon that has this amount of energy:
(Equation 6.2) to calculate the frequency v of a photon that has this amount of energy:
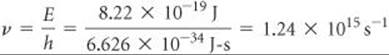
Finally, we use the relationship between frequency and wavelength ![]() (Section 6.1) to calculate the wavelength of the light:
(Section 6.1) to calculate the wavelength of the light:
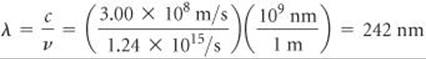
Thus, light of wavelength 242 nm, which is in the ultraviolet region of the electromagnetic spectrum, has sufficient energy per photon to photodissociate an O2 molecule. Because photon energy increases as wavelength decreases, any photon of wavelength shorter than 242 nm will have sufficient energy to dissociate O2.
PRACTICE EXERCISE
The bond energy in N2 is 941 kJ/mol. What is the longest wavelength a photon can have and still have sufficient energy to dissociate N2?
Answer: 127 nm
Fortunately for us, O2 absorbs much of the high-energy, short-wavelength radiation from the solar spectrum before that radiation reaches the lower atmosphere. As it does, atomic oxygen, O, is formed. The dissociation of O2 is very extensive at higher elevations. At 400 km, for example, only 1% of the oxygen is in the form of O2; 99% is atomic oxygen. At 130 km, O2 and atomic oxygen are just about equally abundant. Below 130 km, O2 is more abundant than atomic oxygen because most of the solar energy has been absorbed in the upper atmosphere.
The dissociation energy of N2 is very high, 941 kJ/mol. Analogous to Practice Exercise 18.2, only photons having a wavelength shorter than 127 nm possess sufficient energy to dissociate N2. Furthermore, N2 does not readily absorb photons, even when they possess sufficient energy. As a result, very little atomic nitrogen is formed in the upper atmosphere by photodissociation of N2.
Other photochemical processes besides photodissociation occur in the upper atmosphere, although their discovery has taken many twists and turns. In 1901 Guglielmo Marconi received a radio signal in St. John's, Newfoundland, that had been transmitted from Land's End, England, 2900 km away. Because people at the time thought radio waves traveled in straight lines, they assumed that the curvature of Earth's surface would make radio communication over large distances impossible. Marconi's successful experiment suggested that Earth's atmosphere in some way substantially affects radio-wave propagation. His discovery led to intensive study of the upper atmosphere. In about 1924, the existence of electrons in the upper atmosphere was established by experimental studies.
The electrons in the upper atmosphere result mainly from photoionization, which occurs when a molecule in the upper atmosphere absorbs solar radiation and the absorbed energy causes an electron to be ejected from the molecule. The molecule then becomes a positively charged ion. For photoionization to occur, therefore, a molecule must absorb a photon, and the photon must have enough energy to remove an electron. ![]() (Section 7.4)
(Section 7.4)
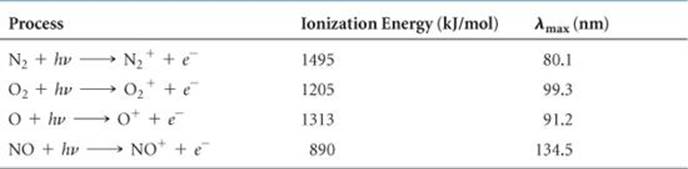
Four important photoionization processes occurring in the atmosphere above about 90 km are shown in ![]() TABLE 18.3. Photons of any wavelength shorter than the maximum lengths given in the table have enough energy to cause photoionization. A look back at Figure 18.3, however, shows you that virtually all of these high-energy photons are filtered out of the radiation reaching Earth because they are absorbed by the upper atmosphere.
TABLE 18.3. Photons of any wavelength shorter than the maximum lengths given in the table have enough energy to cause photoionization. A look back at Figure 18.3, however, shows you that virtually all of these high-energy photons are filtered out of the radiation reaching Earth because they are absorbed by the upper atmosphere.
![]() GIVE IT SOME THOUGHT
GIVE IT SOME THOUGHT
Explain the difference between photoionization and photodissociation.
Ozone in the Stratosphere
Although N2, O2, and atomic oxygen absorb photons having wavelengths shorter than 240 nm, ozone, O3, is the key absorber of photons having wavelengths ranging from 240 to 310 nm, in the ultraviolet region of the electromagnetic spectrum. Ozone in the upper atmosphere protects us from these harmful high-energy photons, which would otherwise penetrate to Earth's surface. Let's consider how ozone forms in the upper atmosphere and how it absorbs photons.
By the time radiation from the Sun reaches an altitude of 90 km above Earth's surface, most of the short-wavelength radiation capable of photoionization has been absorbed. At this altitude, however, radiation capable of dissociating the O2 molecule is sufficiently intense for photodissociation of O2 (Equation 18.1) to remain important down to an altitude of 30 km. In the region between 30 and 90 km, however, the concentration of O2 is much greater than the concentration of atomic oxygen. From this finding, we conclude that the oxygen atoms formed by photodissociation of O2 in this region frequently collide with O2 molecules and form ozone:
TABLE 18.3 • Photoionization Reactions for Four Components of the Atmosphere
![]()
The asterisk on O3 denotes that the molecule contains an excess of energy. This reaction releases 105 kJ/mol. This energy must be transferred away from the O3* molecule quickly or else the molecule will fly apart into O2 and atomic O —a decomposition that is the reverse of the reaction by which O3* is formed.
An energy-rich O3* molecule can release its excess energy by colliding with another atom or molecule and transferring some of the excess energy to it. Let's use M to represent the atom or molecule with which O3* collides. (Usually M is N2 or O2 because these are the most abundant molecules in the atmosphere.) The formation of O3* and the transfer of excess energy to M are summarized by the equations
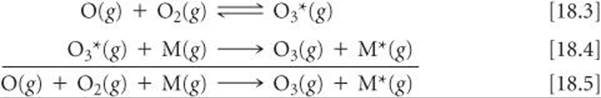
The rate at which the reactions of Equations 18.3 and 18.4 proceed depends on two factors that vary in opposite directions with increasing altitude. First, the Equation 18.3 reaction depends on the presence of O atoms. At low altitudes, most of the radiation energetic enough to dissociate O2 into O atoms has been absorbed; thus, O atoms are more plentiful at higher altitudes. Second, Equations 18.3 and 18.4 both depend on molecular collisions. ![]() (Section 14.5) The concentration of molecules is greater at low altitudes, and so the rates of both reactions are greater at lower altitudes. Because these two reactions vary with altitude in opposite directions, the highest rate of O3 formation occurs in a band at an altitude of about 50 km, near the stratopause (Figure 18.1). Overall, roughly 90% of Earth's ozone is found in the stratosphere.
(Section 14.5) The concentration of molecules is greater at low altitudes, and so the rates of both reactions are greater at lower altitudes. Because these two reactions vary with altitude in opposite directions, the highest rate of O3 formation occurs in a band at an altitude of about 50 km, near the stratopause (Figure 18.1). Overall, roughly 90% of Earth's ozone is found in the stratosphere.
![]() GIVE IT SOME THOUGHT
GIVE IT SOME THOUGHT
Why don't O2 and N2 molecules filter out ultraviolet light with wavelengths between 240 and 310 nm?
The photodissociation of ozone reverses the reaction that forms it. We thus have a cycle of ozone formation and decomposition, summarized as follows:
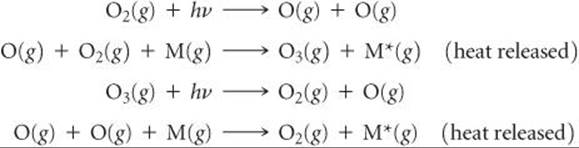
The first and third processes are photochemical; they use a solar photon to initiate a chemical reaction. The second and fourth are exothermic chemical reactions. The net result of the four reactions is a cycle in which solar radiant energy is converted into thermal energy. The ozone cycle in the stratosphere is responsible for the rise in temperature that reaches its maximum at the stratopause (Figure 18.1).
The reactions of the ozone cycle account for some, but not all, of the facts about the ozone layer. Many chemical reactions occur that involve substances other than oxygen. We must also consider the effects of turbulence and winds that mix up the stratosphere. A complicated picture results. The overall result of ozone formation and removal reactions, coupled with atmospheric turbulence and other factors, is to produce the upper-atmosphere ozone profile shown in ![]() FIGURE 18.4, with a maximum ozone concentration occurring at an altitude of about 25 km. This band of relatively high ozone concentration is referred to as the “ozone layer” or the “ozone shield.”
FIGURE 18.4, with a maximum ozone concentration occurring at an altitude of about 25 km. This band of relatively high ozone concentration is referred to as the “ozone layer” or the “ozone shield.”
![]() GO FIGURE
GO FIGURE
Estimate the ozone concentration in moles per liter for the peak value in this graph.
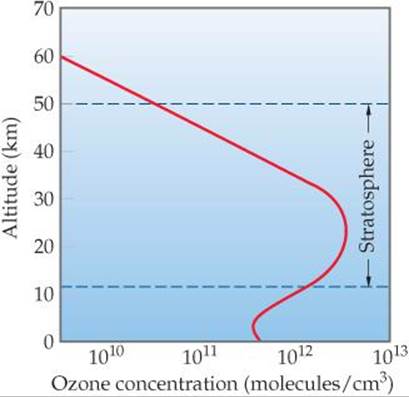
![]() FIGURE 18.4 Variation in ozone concentration in the atmosphere as a function of altitude.
FIGURE 18.4 Variation in ozone concentration in the atmosphere as a function of altitude.
Photons with wavelengths shorter than about 300 nm are energetic enough to break many kinds of single chemical bonds. Thus, the “ozone shield” is essential for our continued well-being. The ozone molecules that form this essential shield against high-energy radiation represent only a tiny fraction of the oxygen atoms present in the stratosphere, however, because these molecules are continually destroyed even as they are formed.